FDA Endorses Xolair as the Sole Treatment for Food Allergies in Both Kids and Grown-ups
Genentech, part of the Roche conglomerate, has made a public announcement stating that the FDA in the United States has sanctioned the use of Xolair (omalizumab) as a therapeutic measure for lessening the severity of hypersensitive responses, such as anaphylactic incidents, which might arise following unintended contact with various food allergens. This approval extends to both adults and children, starting from the age of 1 year, who suffer from food allergies triggered by immunoglobulin E (IgE).
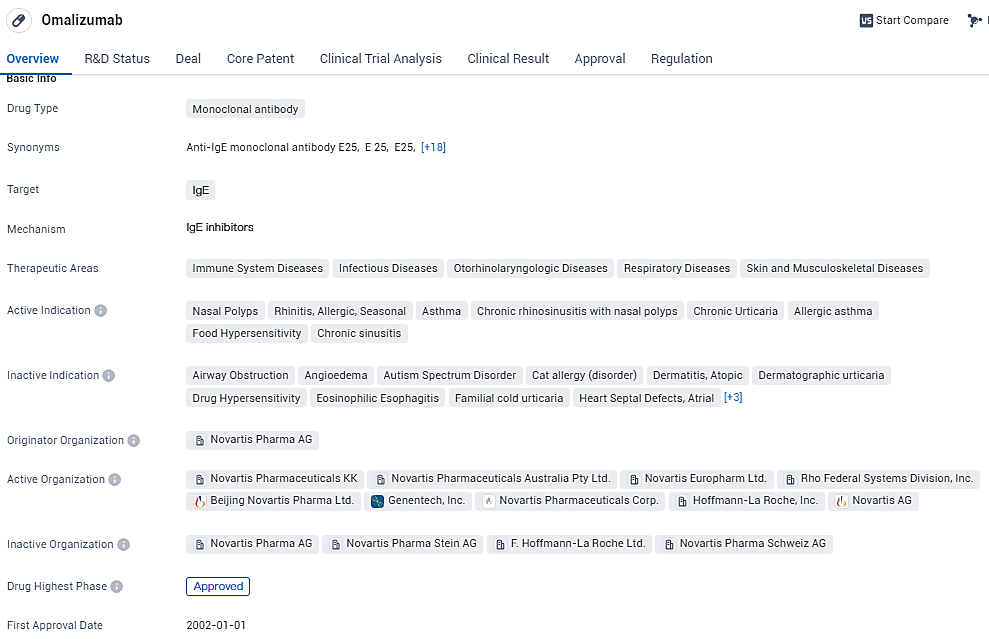
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
It is important to note that Xolair must not be utilized as a rapid response remedy for any forms of acute allergic reactions, including anaphylaxis. Allergies triggered by food involving Immunoglobulin E (IgE) are notably prevalent, usually distinct for their swift symptom onset after exposure to specific food triggers.
As a pioneering medication, Xolair has gained FDA endorsement as the sole treatment designed to minimize the frequency and severity of allergic episodes in individuals diagnosed with one or several food allergies. In the United States, Xolair has now become widely accessible and can be administered to patients deemed suitable for IgE-related food allergy management.
Levi Garraway, M.D., Ph.D., who stands as the Chief Medical Officer and the leader of Global Product Development at Genentech, stated, "Xolair introduces a vital treatment alternative for patients and their families, offering a transformative approach to food allergy management and aiming to mitigate the potentially grave allergic incidents arising from food allergen exposure."
Dr. Garraway further remarked on the regulatory approval: "This milestone is an extension of two decades' worth of patient experiences, building upon a proven track record of efficacy and safety originating from Xolair's initial approval to treat allergic asthma. We anticipate delivering this therapeutic advancement to the food allergy population, who have been in pressing need of such development."
With food allergies increasingly impacting individuals, Sung Poblete, R.N., Ph.D., who serves as the CEO of FARE, emphasized the urgency for innovations in preventing severe allergic reactions and emergencies. As an individual with food allergies, Dr. Poblete expressed her understanding of the profound effect they exert on sufferers and their families, voicing her shared enthusiasm within the community regarding this recent endorsement.
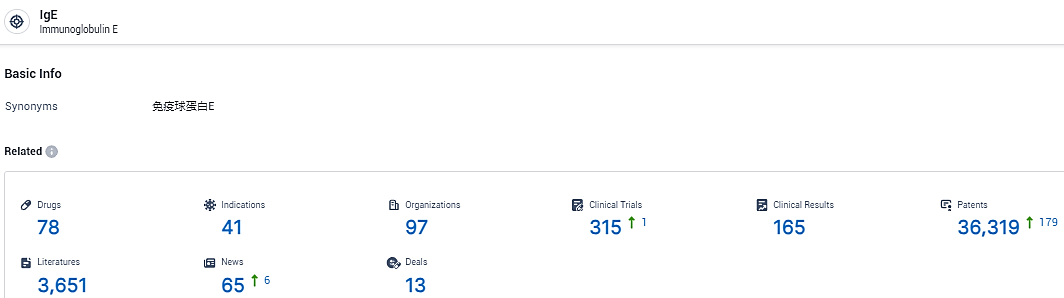
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 78 investigational drugs for the IgE target, including 41 indications, 97 R&D institutions involved, with related clinical trials reaching 315, and as many as 36319 patents.
Xolair is a prescription biologic medicine that is given as an injection under the skin. It is the only FDA-approved antibody designed to target and block IgE — an underlying driver of food allergy reactions. Its approval in multiple countries and regulatory designations highlight its potential as a breakthrough therapy. Omalizumab's mechanism of action and broad therapeutic areas make it a valuable treatment option for patients suffering from conditions such as asthma, allergic rhinitis, and chronic urticaria.