Exploring Growth and Innovation within the Global Antisense Oligonucleotide Therapeutics Market
According to Global Info Research (GIR), the global antisense oligonucleotide (ASO) drug market reached approximately USD 2.8 billion in sales in 2023 and is projected to grow to around USD 10.6 billion by 2030, with a compound annual growth rate (CAGR) of 21.5%. Notably, the global oligonucleotide drug market grew from USD 10 million in 2016 to USD 3.25 billion in 2021, reflecting an impressive CAGR of 217.8%.
As a cutting-edge therapeutic approach, ASOs precisely target the mRNA or pre-mRNA of disease-causing genes, modulating their expression to offer novel solutions for genetic and neurodegenerative diseases and other hard-to-treat conditions. With high specificity and a direct mechanism of action, ASOs not only correct abnormal gene expression but also inhibit harmful protein synthesis at the source, demonstrating significant potential for clinical applications. Advances in technology and ongoing clinical trials are driving ASO therapies towards maturity, heralding a new era of precision medicine focused on gene regulation.
Factors driving ASO market growth include:
·Increased Research and Development: A surge in R&D activities related to gene therapy and personalized medicine has fueled demand for ASO technology. With advancements in genomics, scientists are gaining a better understanding of gene expression regulation mechanisms and can utilize ASO technology to precisely target specific gene sequences.
·Rising Incidence of Genetic Disorders: The growing prevalence of genetic disorders has increased demand for effective treatments, and ASO technology offers promising options. For example, in the case of genetic conditions like Duchenne muscular dystrophy (DMD), ASOs can restore the production of functional proteins by altering mRNA splicing.
·Advances in Drug Delivery: Progress in ASO delivery methods, including peptide carriers and lipid nanoparticles, has simplified ASO therapy administration. These delivery technologies not only enhance ASO stability and bioavailability but also reduce side effects, improving therapeutic efficacy.
·Supportive Regulatory Environment: Regulatory agencies are increasingly supportive of innovative gene therapies, creating favorable conditions for ASO drug development. For instance, both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established pathways to expedite the approval process for rare and orphan drugs.
·Strategic Collaborations: Collaborations between biotech firms, major pharmaceutical companies, and research institutions promote innovation and accelerate ASO therapy development. These partnerships facilitate resource sharing, expertise exchange, and technology advancements, speeding the transition of drugs from the lab to the market.
How Does ASOs Work?
ASOs play a vital role in gene expression regulation through several mechanisms:
·Gene Targeting: ASOs are designed to target specific genes or mRNA molecules. Each ASO molecule is complementary to a particular mRNA sequence, enabling it to bind and interact with the target mRNA.
RNase H-Mediated mRNA Degradation: Once inside the cell, the ASO binds to the target mRNA. This binding recruits RNase H, which degrades the mRNA paired with the ASO, leading to mRNA breakdown.
Complementary Base Pairing: The ASO’s guide strand binds to complementary mRNA through base pairing. This binding is highly specific and only occurs when there is a perfect or near-perfect match between the ASO and the target mRNA.
·mRNA Degradation: The ASO-mRNA binding triggers RNase H to cleave the mRNA at the interaction site. This cleavage prevents the mRNA from being translated into functional proteins.
·Gene Expression Silencing: As mRNA is degraded, the cell cannot produce the corresponding protein. This effectively silences the gene’s expression, reducing or entirely stopping the production of the protein encoded by the gene.
·Temporary Effect: ASO-mediated gene silencing is typically temporary. As the affected mRNA degrades and cellular machinery continues to function, gene expression can return to normal once the ASO is no longer present.
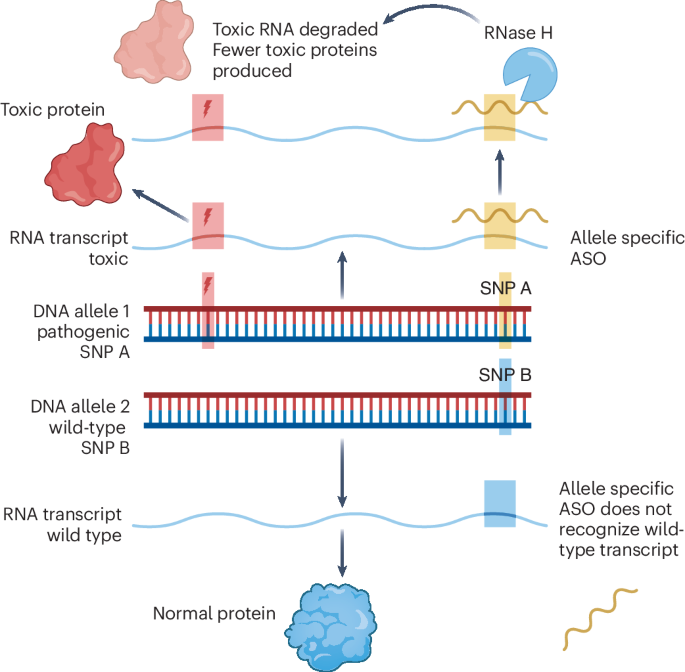
In addition, ASOs can modify pre-mRNA splicing through steric hindrance, thus regulating gene translation. These combined mechanisms make ASOs a powerful gene expression regulation tool suitable for treating various diseases, including but not limited to genetic disorders, cancer, infectious disease prevention, and neurological disease treatment.
ASO Drug Segmentation Trends
Due to their high molecular weight and charged properties, ASOs have difficulty naturally crossing cell membranes in the body, thus requiring specialized delivery systems. Below are some main classifications of ASO delivery methods:
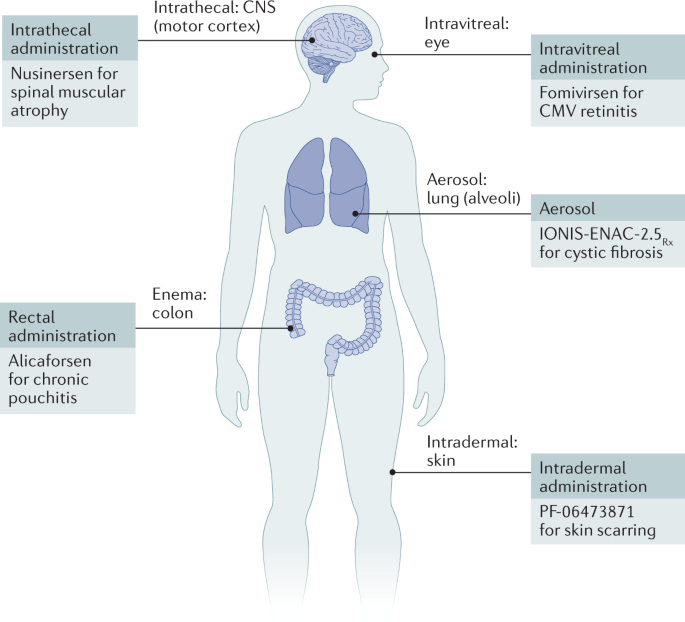
1.Naked Oligonucleotides:
·Direct Administration: Unmodified ASOs are directly administered via injection or other methods, typically at higher doses due to susceptibility to enzymatic degradation and challenges with cellular uptake.
2.Chemical Modifications:
·Modified Oligonucleotides: Chemical modifications enhance ASO stability, such as 2'-O-methylation, phosphorothioate (PS) modification, and locked nucleic acid (LNA) modifications, which improve stability, reduce degradation, and increase cellular uptake efficiency.
3.Ligand Conjugation:
·GalNAc Conjugation: Conjugating ASOs with GalNAc (N-acetylgalactosamine) ligands enhances selective delivery to liver cells, a method used in Alnylam Pharmaceuticals' products.
·Other Ligands: Various ligands are used to target delivery to specific cells or tissues.
4.Nanoparticles:
·Lipid Nanoparticles (LNPs): Encapsulating ASOs within LNPs protects them from degradation and aids cellular entry. LNPs can circulate through the bloodstream to target organs or tissues and enter cells via endocytosis.
·Polymer Nanoparticles: Using polymer matrices to encapsulate ASOs also enhances stability and internalization efficiency.
5.Exosomes:
·Exosome Delivery: Using naturally secreted vesicles (exosomes) as carriers delivers ASOs to target cells, reducing immune responses and enhancing specificity.
6.Viral Vectors:
·Adeno-Associated Virus (AAV): Though primarily used in gene therapy, AAV may also be utilized to deliver ASOs in certain cases.
7.Localized Delivery:
·Direct Injection: Directly injecting ASOs into the affected area, such as muscle or the central nervous system, is suitable for treating diseases localized to specific organs or tissues.
·Inhalation Therapy: For respiratory diseases, ASOs can be delivered to the lungs via inhalation.
Each delivery method has unique advantages and limitations, and selecting the appropriate system depends on the therapeutic target, disease type, and ASO's chemical properties. With technological advancements, new delivery platforms and methods are emerging, aiming to improve the efficacy and safety of ASO therapies.
ASO Applications by Indication
1.Genetic Disorders:
·DMD: Drugs like Exondys 51 and Vyondys 53 from Sarepta Therapeutics, and Roche's Ryonspurt (Golodirsen), facilitate exon skipping to restore functional dystrophin protein synthesis.
·Spinal Muscular Atrophy (SMA): Spinraza (Nusinersen) from Biogen modifies SMN2 gene splicing to increase SMN protein levels.
2.Neurological Diseases:
·Familial Amyloid Polyneuropathy (FAP): Drugs like AstraZeneca’s Onpattro (Patisiran) and Takhzyro (Lanadelumab), the former using RNAi to reduce TTR protein levels, while the latter, a monoclonal antibody, prevents hereditary angioedema attacks.
·Amyotrophic Lateral Sclerosis (ALS): Tofersen from Biogen targets mutant SOD1 protein reduction for treating SOD1 gene mutation-associated ALS.
3.Metabolic Disorders:
·Transthyretin Amyloidosis (ATTR): Tegsedi (Inotersen) by Akcea Therapeutics and Ionis Pharmaceuticals, and Vyndaqel (Tafamidis) by Pfizer, reduce circulating TTR levels or stabilize the TTR tetramer to prevent amyloid fiber formation.
·Familial Hypercholesterolemia (FH): Waylivra (Volanesorsen) from Akcea Therapeutics and Ionis Pharmaceuticals lowers lipoprotein(a) levels for treating homozygous familial hypercholesterolemia (HoFH).
4.Viral Infections:
·Chronic Hepatitis B (CHB): Viread (Tenofovir Disoproxil Fumarate) and Hepcludex (Bulevirtide) from Gilead Sciences, with the former suppressing viral replication and the latter as the first approved ASO for chronic hepatitis B treatment.
·Cytomegalovirus Infection (CMV): Vitravene (Fomivirsen) from Isis Pharmaceuticals, treats CMV retinitis in immunocompromised individuals.
5.Cancer:
·Hematologic Malignancies: Revlimid (Lenalidomide) from Celgene and Erwinaze (Erwinia L-asparaginase) from Jazz Pharmaceuticals, with the former treating multiple myeloma and the latter acute lymphoblastic leukemia (ALL).
6.Cardiovascular Diseases:
·Heart Failure (HF): Cardior Pharmaceuticals is developing RNA-based therapies to restore myocardial function by inhibiting non-coding RNAs (ncRNAs).
7.Other Rare Diseases:
·Homozygous Familial Hypercholesterolemia (HoFH): Waylivra (Volanesorsen) from Akcea Therapeutics and Ionis Pharmaceuticals lowers lipoprotein(a) levels to treat HoFH.
Regional Analysis of ASO Development
ASOs are experiencing robust growth globally, with North America and Europe leading the way due to their advanced technologies and regulatory frameworks. Meanwhile, the Asian market, especially China, is rapidly emerging as a significant driver of global ASO drug development, fueled by its vast demand potential and increasing innovation capacity.
In recent years, the ASO industry has been undergoing a global transformation with notable growth momentum, particularly in the North American market. For a time, market control was firmly held by the two major players, Sarepta and Ionis. However, 2023 marked a turning point. A wave of new oligonucleotide drugs gained approval, reshaping the competitive landscape. In May 2023, Tofersen, an ASOs co-developed by Biogen and Ionis, received regulatory approval. This was followed by the December approval of Eplontersen, an ASO therapy introduced by AstraZeneca in collaboration with Ionis. Concurrently, Novo Nordisk's RNAi therapy Nedosiran was successfully launched in September, and Astellas's nucleic acid aptamer drug Izervay for geographic atrophy received approval in August. This shift in approvals has gradually disrupted the Sarepta-Ionis market duopoly.
Key Players in the ASO Development Market
1.Ionis Pharmaceuticals
Ionis Pharmaceuticals is a pioneer in the field of ASO therapies and remains at the forefront of ASO development. Since its founding in 1989, Ionis has led the ASO sector with a core platform that includes ligand-conjugated antisense technology (LICA), allowing targeted delivery of drugs to specific cells and tissues by conjugating ligands with specific cell surface receptors. As of 2023, Ionis has developed seven ASO products, collaborating with various pharmaceutical companies for late-stage drug development.
2.Sarepta Therapeutics
A leader in the DMD space, Sarepta Therapeutics actively participates in ASO discovery and development, focusing on muscle-related diseases. The company has achieved FDA approval for four DMD drugs, including Eteplirsen, Golodirsen, Casimersen, and Elevidys. The first three are ASOs, while Elevidys is a gene therapy. Sarepta’s core platform utilizes phosphorodiamidate morpholino oligomer (PMO) technology to enable exon skipping, allowing pre-mRNA to bypass mutated exons during translation to restore functional dystrophin protein, alleviating symptoms of DMD.
3.Biogen
Biogen focuses on developing ASO therapies for neurological disorders. In collaboration with Ionis, Biogen developed Nusinersen (marketed as SPINRAZA), the world’s first ASO drug for treating spinal muscular atrophy (SMA). Since its launch in 2016, Nusinersen’s sales have approached $10 billion, demonstrating ASO’s vast market potential. Biogen and Ionis’s joint development of Qalsody (tofersen), approved by the FDA in April 2023, treats amyotrophic lateral sclerosis (ALS) in adults with SOD1 gene mutations, marking the fourth new drug approved for ALS globally.
Recent Innovations in ASO Development
The ASO field has seen significant advancements recently, such as the approval of Tofersen by Biogen and Ionis for specific types of ALS and Eplontersen by AstraZeneca and Ionis for treating transthyretin amyloidosis (ATTR). These developments not only expand therapeutic options but also offer new hope for patients with rare and severe diseases. Moreover, companies like Ausper Biopharma and SicaGene are advancing their own ASO pipelines, further enriching the field and highlighting ASO technology's potential in precision medicine.
Conclusion
ASOs have shown tremendous potential as an emerging treatment modality within precision medicine. By targeting disease-causing mRNA, ASOs can effectively inhibit harmful protein synthesis or correct faulty splicing, providing novel therapeutic avenues for genetic disorders, neurodegenerative diseases, and certain cancer types. In recent years, successful market entries of drugs like Tofersen and Eplontersen have not only demonstrated ASO therapies' efficacy and safety in clinical applications but also signal the arrival of more innovative drugs that will benefit a broader patient population. These advancements signify the maturation of ASO technology in biomedical research, injecting fresh momentum into the healthcare industry and ushering in a new era of gene-regulation-centered therapies.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
- 1. O'Leary, K., Guiding the development of individualized ASO therapies. Nat Med, 2023.
- 2. Schuele, R., et al., Advancing ASO therapies from development to implementation. Nat Med, 2024. 30(10): p. 2725-2726.
- 3. Crooke, S.T., et al., Antisense technology: an overview and prospectus. Nat Rev Drug Discov, 2021. 20(6): p. 427-453.