Jubilant Therapeutics Begins Global Trials for Cancer Drugs JBI-802 and JBI-778
Jubilant Therapeutics Inc., a biopharmaceutical firm focused on developing small molecule precision therapeutics to tackle unmet medical requirements in oncology and autoimmune disorders, has announced the initiation of dosing for the first patients in international clinical trials related to two of its pipeline programs. These include the Phase I/II clinical trial of JBI-802 for heme-oncology and the Phase I clinical trial of JBI-778 for solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
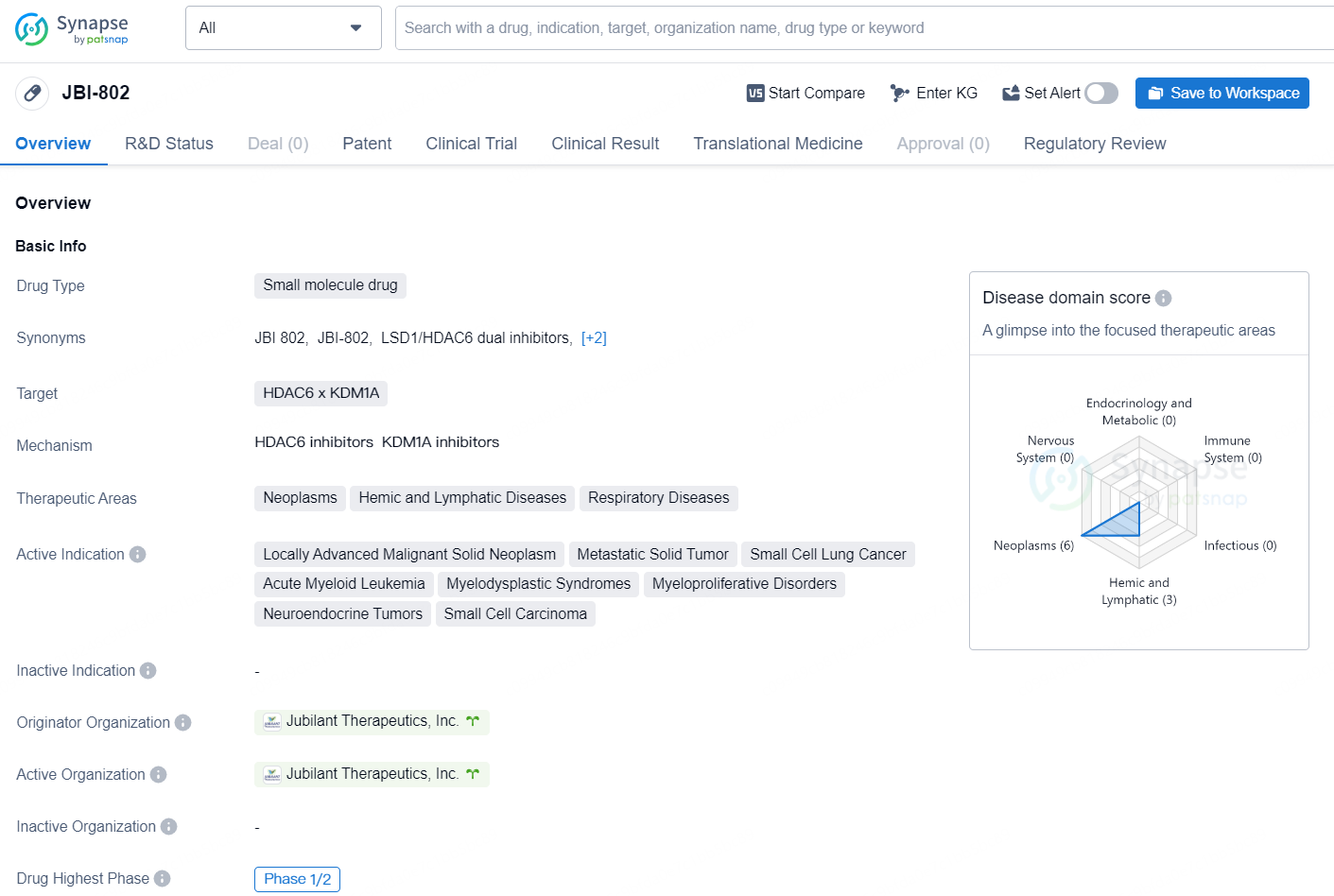
JBI-802 represents an innovative oral small-molecule that functions as a dual inhibitor of LSD1 (Lysine-specific histone demethylase 1A) and HDAC6 (Histone deacetylase 6) within the CoREST (Co-repressor of Repressor Element-1 Silencing Transcription) complex. In an earlier Phase I trial involving patients with advanced solid tumors, JBI-802 demonstrated a dose-dependent increase in exposure levels across different cohorts, showing a significant relationship between exposure and the target effect of reduced platelet counts. Notably, there were no incidences of Dysgeusia or Anemia, which are commonly associated with LSD1 inhibitors. Furthermore, the Phase I study indicated antitumor activity in Non-Small Cell Lung Cancer (NSCLC) patients, including a verified partial response. The findings indicate potential for further development of JBI-802 in treating Essential Thrombocythemia and Myelodysplastic Syndrome/Myeloproliferative Neoplasms (MDS/MPN) with elevated platelet counts.
Essential Thrombocythemia is a chronic condition characterized by an overproduction of platelets, affecting more than 100,000 individuals in the U.S., with hydroxyurea being the main treatment option, though it presents substantial challenges in terms of safety and efficacy for patients.
The next clinical trial will evaluate the safety profile and establish the recommended Phase II dosage for JBI-778, which is an oral inhibitor of PRMT5 (Protein arginine N-methyltransferase 5) that can penetrate the brain. This trial will focus on NSCLC patients with resistance to mEGFR Tyrosine Kinase Inhibitors (TKIs), as well as those with IDH+ high-grade glioma (HGG) and Adenoid Cystic Carcinoma (ACC).
While PRMT5 has been identified as a significant pathway in various cancers, efforts in drug development have yielded inconsistent outcomes, largely due to safety issues related to inhibiting PRMT5 via a SAM competitive model, alongside limited patient applicability associated with MTAP-null tumors. JBI-778 is a novel substrate-competitive PRMT5 inhibitor designed for effective brain penetration, which has not exhibited adverse effects in preclinical tests. It is capable of targeting both MTAP-null and wild type tumors, including brain tumors, thus widening the patient population eligible for treatment, particularly those with brain metastases.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
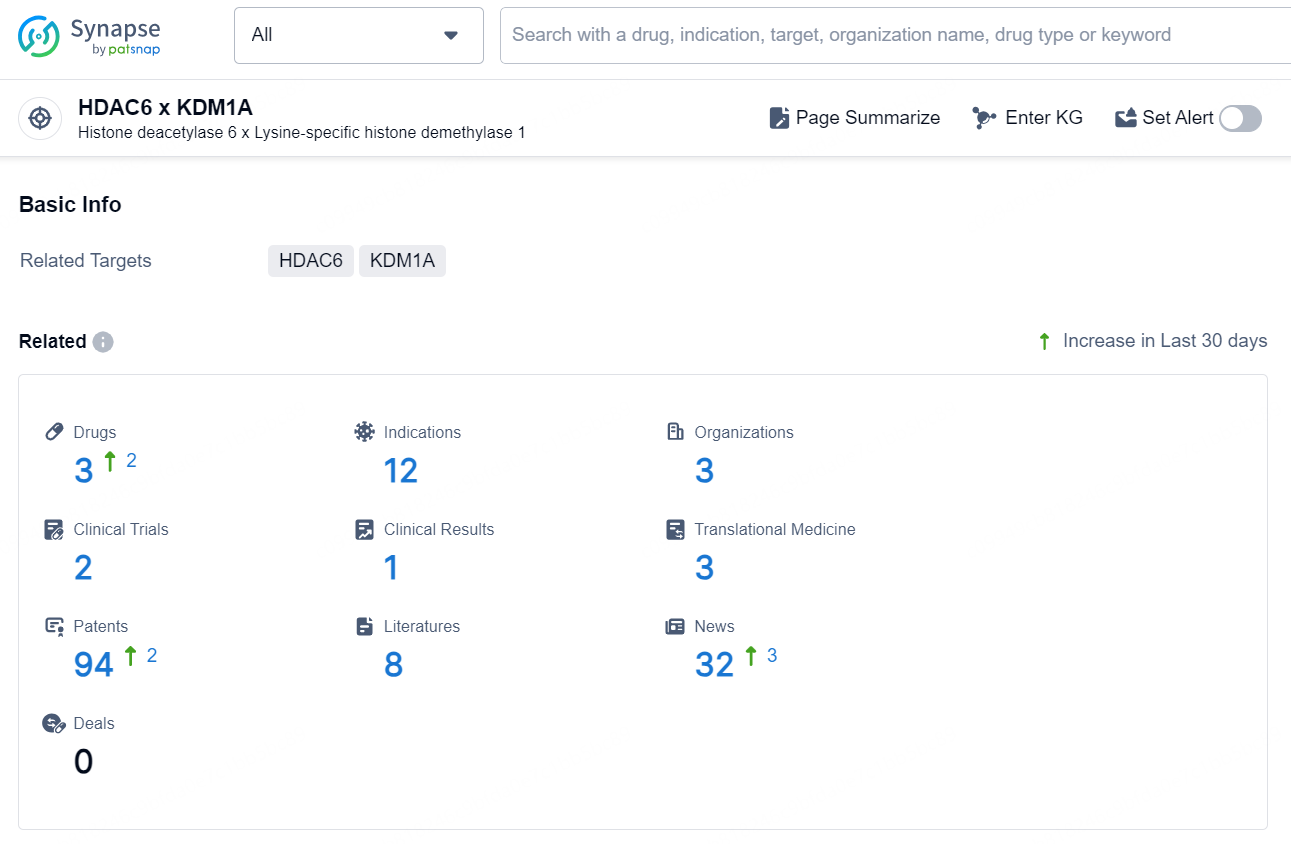
According to the data provided by the Synapse Database, As of October 29, 2024, there are 3 investigational drugs for the HDAC6 and KDM1A targets, including 12 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 94 patents.
JBI-802 is a small molecule drug developed by Jubilant Therapeutics, Inc., with a primary focus on targeting HDAC6 and KDM1A. The therapeutic areas for this drug include neoplasms, hemic and lymphatic diseases, and respiratory diseases. The active indications for [JBI-802] are locally advanced malignant solid neoplasm, metastatic solid tumor, small cell lung cancer, acute myeloid leukemia, myelodysplastic syndromes, myeloproliferative disorders, neuroendocrine tumors, and small cell carcinoma.