Maze Therapeutics Reports Positive Phase 1 Results for MZE829 in Treating APOL1 Kidney Disease
Maze Therapeutics, a biopharmaceutical firm in the clinical development phase that utilizes human genetics to create innovative small molecule precision therapies for individuals with prevalent diseases, has reported encouraging outcomes from the Phase 1 clinical trial of MZE829 involving healthy participants. MZE829 is an oral small molecule that functions as an inhibitor of APOL1, which Maze is progressing as a potential solution for individuals suffering from APOL1 kidney disease (AKD), a specific form of chronic kidney disease that is estimated to impact more than one million people in the United States. The APOL1 protein is produced by the APOL1 gene in humans, and genetic variations such as G1 and G2 are linked to a greater likelihood of developing various progressive kidney conditions, particularly in individuals of West African descent. MZE829 was discovered through insights derived from Maze’s exclusive, specially designed platform, Maze Compass™.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
A first-in-human Phase 1 randomized, placebo-controlled trial involving both single and multiple ascending doses was conducted to assess the safety, pharmacokinetics (PK), dietary impact, and potential interactions of the oral medication MZE829 in healthy participants, comprising a total of 111 subjects. Findings indicated that MZE829 was well tolerated, with single doses reaching up to 480 mg and multiple doses up to 350 mg, with all reported treatment-related adverse events classified as mild; no severe or serious adverse events were noted. PK data showed a dose-proportional response with minimal variability among doses. The drug exhibited an approximate half-life of 15 hours, indicating that MZE829 could be administered on a once-daily schedule. The assessment of potential drug interactions suggested that MZE829 may be safely combined with standard care medications for patients with acute kidney disease (AKD), such as cyclosporine and tacrolimus.
“We are very pleased with the positive outcome of our Phase 1 trial of MZE829, demonstrating its tolerability and establishing the dosing regimen that we plan to take into our Phase 2 trial in patients with AKD,” stated Harold Bernstein, M.D., Ph.D., president of R&D and chief medical officer at Maze. “Based on its mechanism targeting APOL1, as well as genetics data derived through our Compass platform, we believe MZE829 can benefit a large segment of patients with AKD. Consequently, we aim to recruit patients with diverse characteristics for our Phase 2 study to capture the full range of AKD patients and identify those who may gain from MZE829 treatment.”
The firm plans to execute the Phase 2 clinical trial using an open-label basket design that includes various clinical phenotypes and moderate to severe disease forms based on proteinuria levels. This trial will focus on AKD patients with two copies of the high-risk APOL1 alleles (G1 and G2) and will assess effectiveness through uACR reduction in a broad AKD population. The company is looking to commence the Phase 2 study in the first quarter of 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
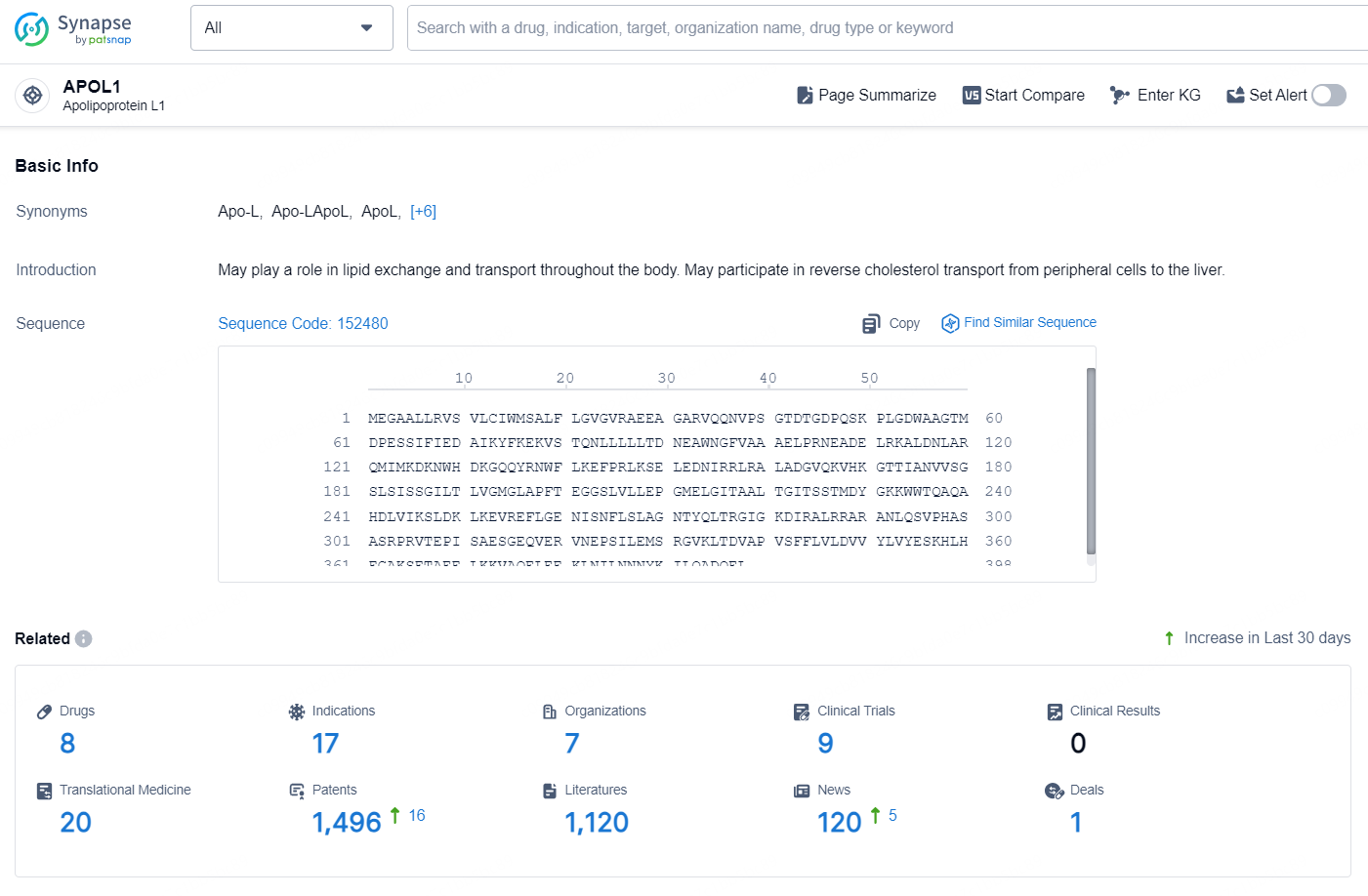
According to the data provided by the Synapse Database, As of October 29, 2024, there are 8 investigational drugs for the APOL1 targets, including 17 indications, 7 R&D institutions involved, with related clinical trials reaching 9, and as many as 1496 patents.
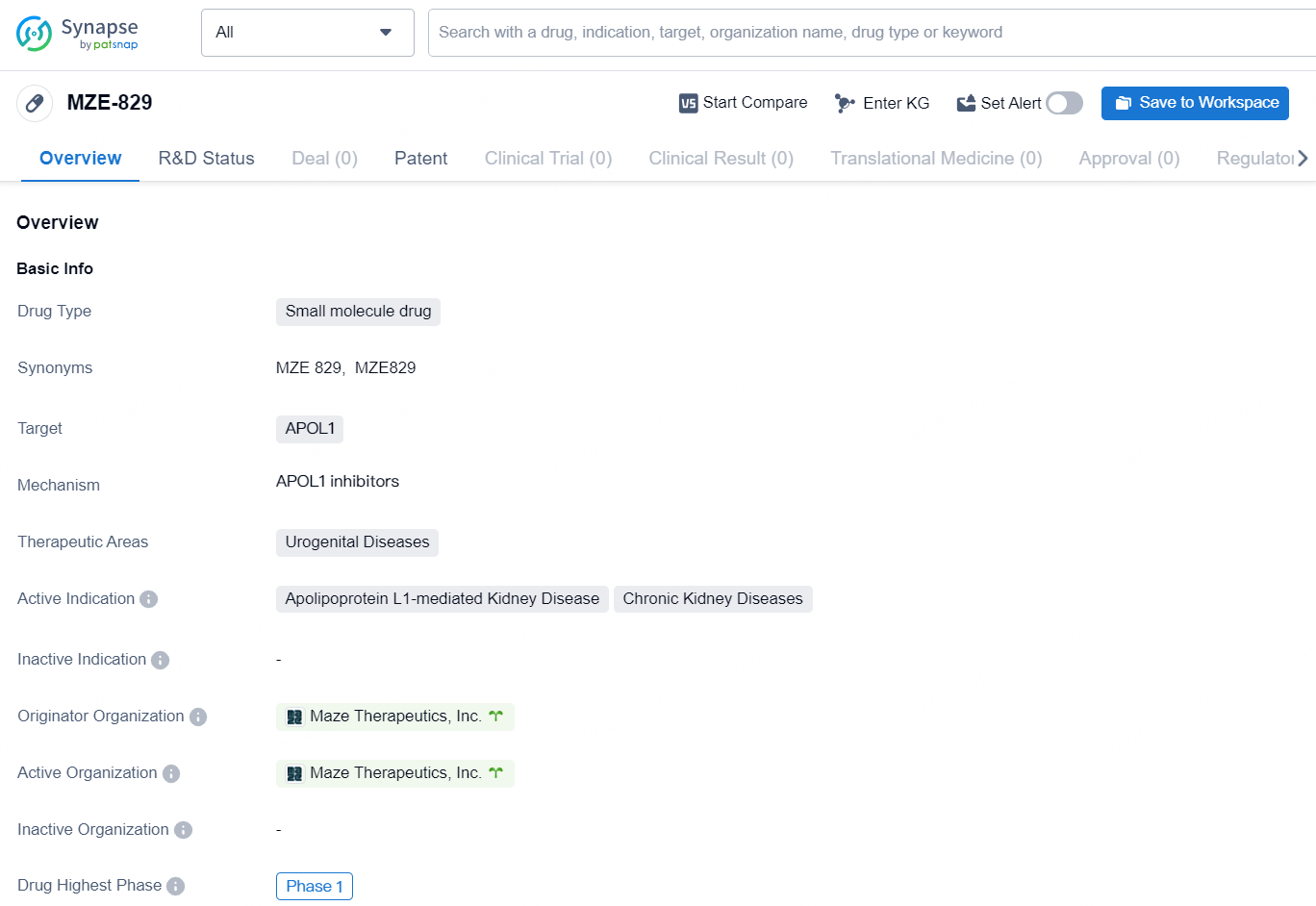
MZE-829 is a small molecule drug developed by Maze Therapeutics, Inc. that targets APOL1. This drug is specifically intended for the treatment of urogenital diseases, with a focus on Apolipoprotein L1-mediated Kidney Disease and Chronic Kidney Diseases. As of the latest information available, the drug is in its highest phase globally, which is Phase 1.