Exploring Ibandronate sodium's Revolutionary R&D Successes
Ibandronate sodium's R&D Progress
Ibandronate Sodium is a small molecule drug that is primarily used to target the FDPS enzyme. It is indicated for the treatment of various conditions within the therapeutic areas of Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, and Other Diseases. The drug is specifically approved for the treatment of Spinal Fractures, Osteoporosis, Osteoporosis in postmenopausal women, Fractures, Spontaneous, and Hypercalcemia.
The originator organization of Ibandronate Sodium is Genentech, Inc., a renowned pharmaceutical company. And the highest R&D phase of this drug is approved.
Ibandronate Sodium received its first approval in June 1996 in the European Union. This suggests that it was initially launched and made available to patients in this region. The drug's approval in the European Union serves as an important milestone in its development and signifies its compliance with the regulatory standards set by the European Medicines Agency (EMA).
The therapeutic areas targeted by Ibandronate Sodium highlight its potential in addressing various medical conditions related to endocrinology, metabolic diseases, skin, and musculoskeletal disorders. The drug's active indications, such as Spinal Fractures, Osteoporosis, and Hypercalcemia, indicate its effectiveness in treating these specific conditions.
As a small molecule drug, Ibandronate Sodium is likely to have a well-defined chemical structure, allowing for precise targeting of the FDPS enzyme. This characteristic may contribute to its efficacy and specificity in treating the indicated diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ibandronate sodium: FDPS inhibitors
FDPS inhibitors are a type of drug that target and inhibit the activity of the enzyme farnesyl diphosphate synthase (FDPS). FDPS is an important enzyme involved in the mevalonate pathway, which is responsible for the synthesis of isoprenoids, including cholesterol and other essential molecules in the body.
From a biomedical perspective, FDPS inhibitors have potential therapeutic applications in various diseases, particularly in the treatment of cancer. By inhibiting FDPS, these inhibitors can disrupt the production of isoprenoids, which are crucial for cell growth and proliferation. In cancer cells, excessive isoprenoid production is often observed, contributing to uncontrolled cell growth. Therefore, FDPS inhibitors can help to selectively target and inhibit the growth of cancer cells while sparing normal cells.
FDPS inhibitors may also have implications in other conditions such as bone disorders, as isoprenoids play a role in bone metabolism. Additionally, these inhibitors have been studied for their potential anti-inflammatory effects.
It is important to note that FDPS inhibitors are still under investigation and not yet approved for widespread clinical use. Further research and clinical trials are needed to determine their safety, efficacy, and potential side effects.
Drug Target R&D Trends for Ibandronate sodium
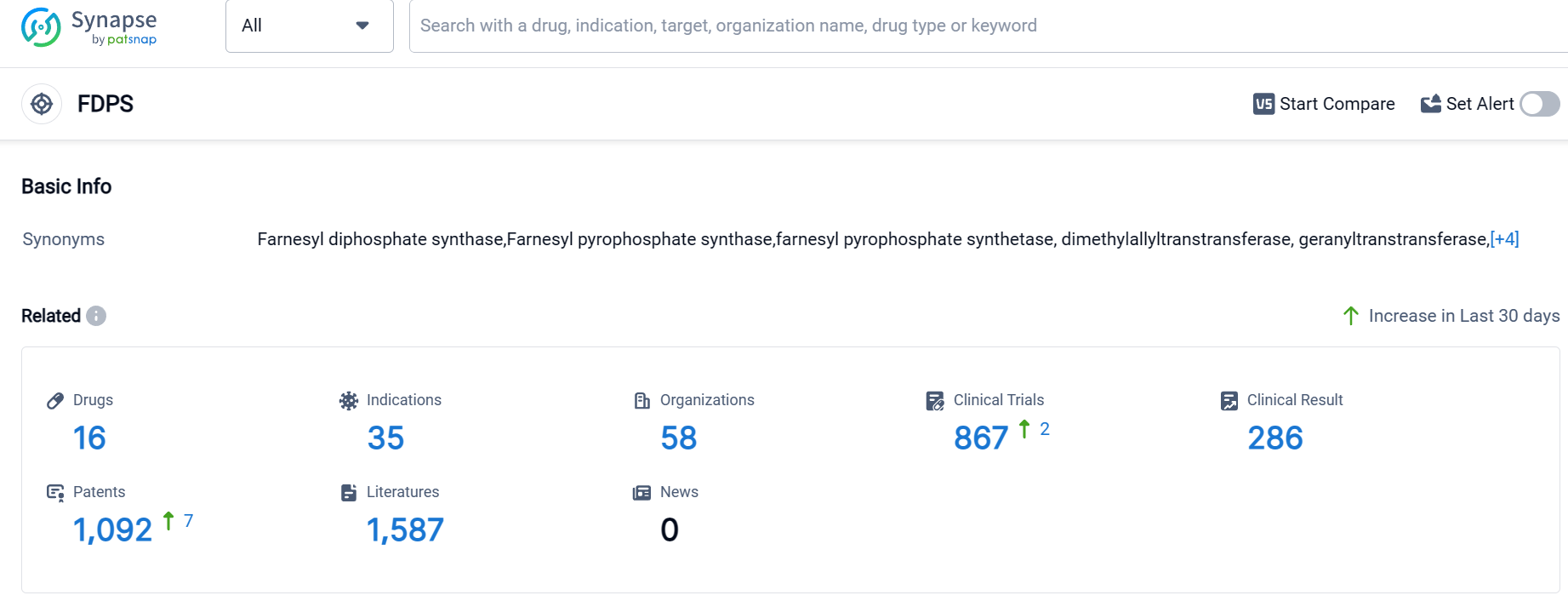
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 16 FDPS drugs worldwide, from 58 organizations, covering 35 indications, and conducting 867 clinical trials.
The analysis of the target FDPS reveals a competitive landscape with multiple companies focusing on the development of drugs. Novartis AG and Teva Pharmaceutical Industries Ltd. are leading in terms of the highest number of approved drugs. The indication analysis highlights a strong focus on bone-related diseases and conditions, with Osteoporosis being the most common indication. Small molecule drugs are progressing rapidly, indicating a focus on traditional drug development. The analysis of countries/locations shows progress in the European Union, Japan, China, the United States, and other countries. China's progress in the target FDPS is particularly noteworthy. Overall, the target FDPS presents opportunities for further development and competition in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ibandronate Sodium is a small molecule drug developed by Genentech, Inc. It has received global approvals, with its first approval in the European Union in 1996. The drug targets the FDPS enzyme and is indicated for the treatment of various conditions within the therapeutic areas of Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, and Other Diseases. Its active indications include Spinal Fractures, Osteoporosis, Osteoporosis in postmenopausal women, Fractures, Spontaneous, and Hypercalcemia.





