Estradiol valerate: Detailed Review of its Transformative R&D Success
Estradiol valerate's R&D Progress
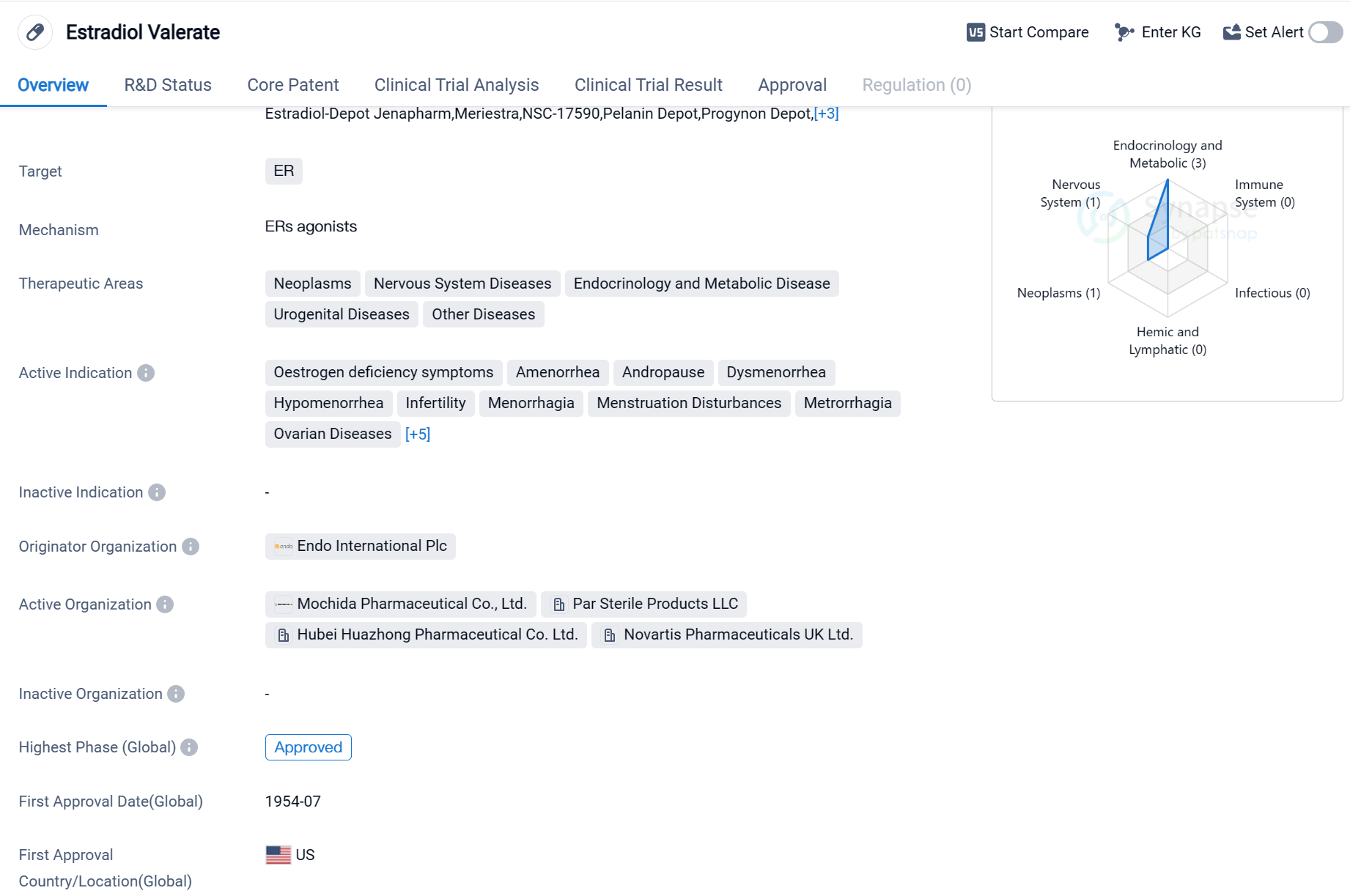
Estradiol Valerate is a small molecule drug that primarily targets the estrogen receptor (ER). It is used in the treatment of various therapeutic areas including neoplasms, nervous system diseases, endocrinology and metabolic diseases, urogenital diseases, and other diseases. The drug is indicated for the management of symptoms related to estrogen deficiency such as oestrogen deficiency symptoms, amenorrhea, andropause, dysmenorrhea, hypomenorrhea, infertility, menorrhagia, menstruation disturbances, metrorrhagia, ovarian diseases, uterine diseases, hypoestrogenism, prostatic cancer, vasomotor symptoms, and vulvar and vaginal atrophy.
Estradiol Valerate was first approved in the United States in July 1954, making it one of the earliest drugs in its class to receive regulatory approval. The drug is developed and marketed by Endo International Plc, a pharmaceutical company specializing in various therapeutic areas.
The highest R&D phase of this drug is approved globally.
The approval of Estradiol Valerate in China further highlights its global reach and acceptance in different markets. This suggests that the drug has met the necessary regulatory requirements and has been deemed suitable for use in the Chinese healthcare system.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Estradiol valerate: ERs agonists
ERs agonists refers to compounds or substances that activate the estrogen receptors (ERs) in the body. Estrogen receptors are proteins found in various tissues, including the reproductive organs, bones, and brain, that bind to estrogen hormones. When activated by agonists, such as certain drugs or natural compounds, ERs initiate a cascade of cellular responses.
From a biomedical perspective, ERs agonists can have therapeutic applications in conditions related to estrogen signaling, such as hormone replacement therapy, menopausal symptoms, and certain types of cancer. By activating ERs, these agonists can mimic the effects of estrogen, leading to specific physiological responses in target tissues.
It is important to note that the specific effects and applications of ERs agonists can vary depending on the compound or substance used. Different agonists may have varying affinities for the different subtypes of estrogen receptors (ERα and ERβ), leading to distinct biological responses. Additionally, the duration and intensity of ER activation can also influence the overall physiological outcomes.
Drug Target R&D Trends for Estradiol valerate
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 484 ER drugs worldwide, from 442 organizations, covering 203 indications, and conducting 3876 clinical trials.
The current competitive landscape of target ER is characterized by the dominance of companies like Bayer AG, Pfizer Inc., and Mochida Pharmaceutical Co., Ltd. These companies have shown significant growth and have made substantial progress in R&D. Indications such as contraception, osteoporosis (postmenopausal), and breast cancer have seen the highest number of approved drugs, indicating a focus on addressing these medical needs. Small molecule drugs and PROTACs are the drug types progressing most rapidly, suggesting intense competition and innovation. The United States, China, and the European Union are the countries/locations developing fastest, with China showing notable progress in drug development. Overall, the target ER presents a competitive landscape with promising future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Estradiol Valerate is a well-established drug in the pharmaceutical industry, with a long history of use and regulatory approval. Its wide range of therapeutic indications reflects its versatility in addressing various medical conditions related to estrogen deficiency. As an expert in the pharmaceutical industry, it is important to objectively present this information without adding fictional data or subjective interpretations.