PLB-1004: A Quick Look at Its R&D Progress and Clinical Results from the 2023
With the disclosure of the interim results of a first-in-human, dose escalation and expansion study of PLB-1004 at the 2023 AACR Congress, a new light has been shed on its potential benefits in the clinical setting.
PLB-1004's R&D Progress
PLB-1004 is a small molecule drug that falls under the category of biomedicine. It specifically targets EGFR exon 20 and HER2 exon 20. The drug is primarily focused on treating neoplasms and respiratory diseases.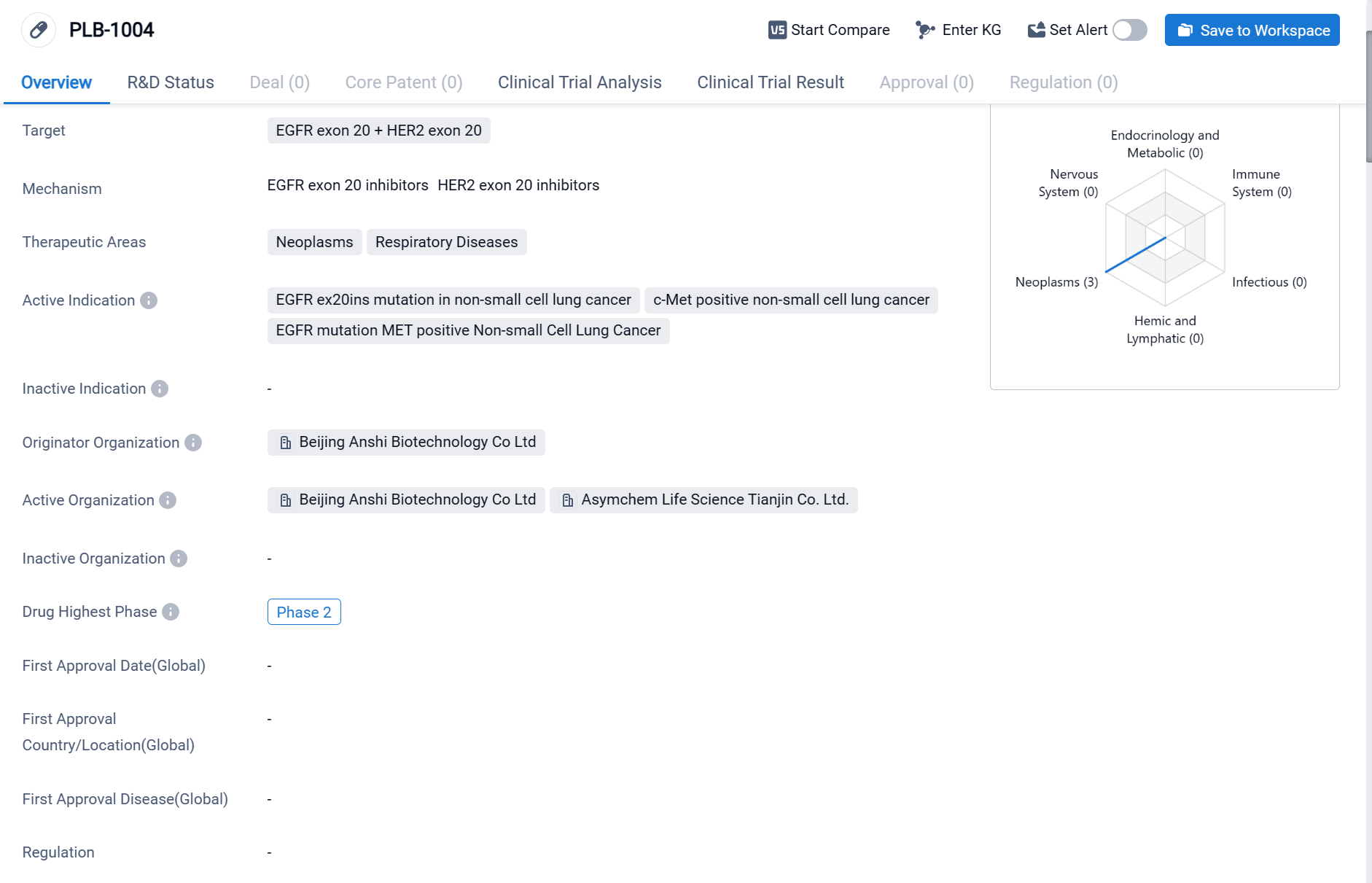
According to the Patsnap Synapse, PLB-1004 has reached Phase 2, which is the highest phase globally. And the clinical trial areas for PLB-1004 are primarily in the United States and China. The key indication is Non-Small Cell Lung Cancer. 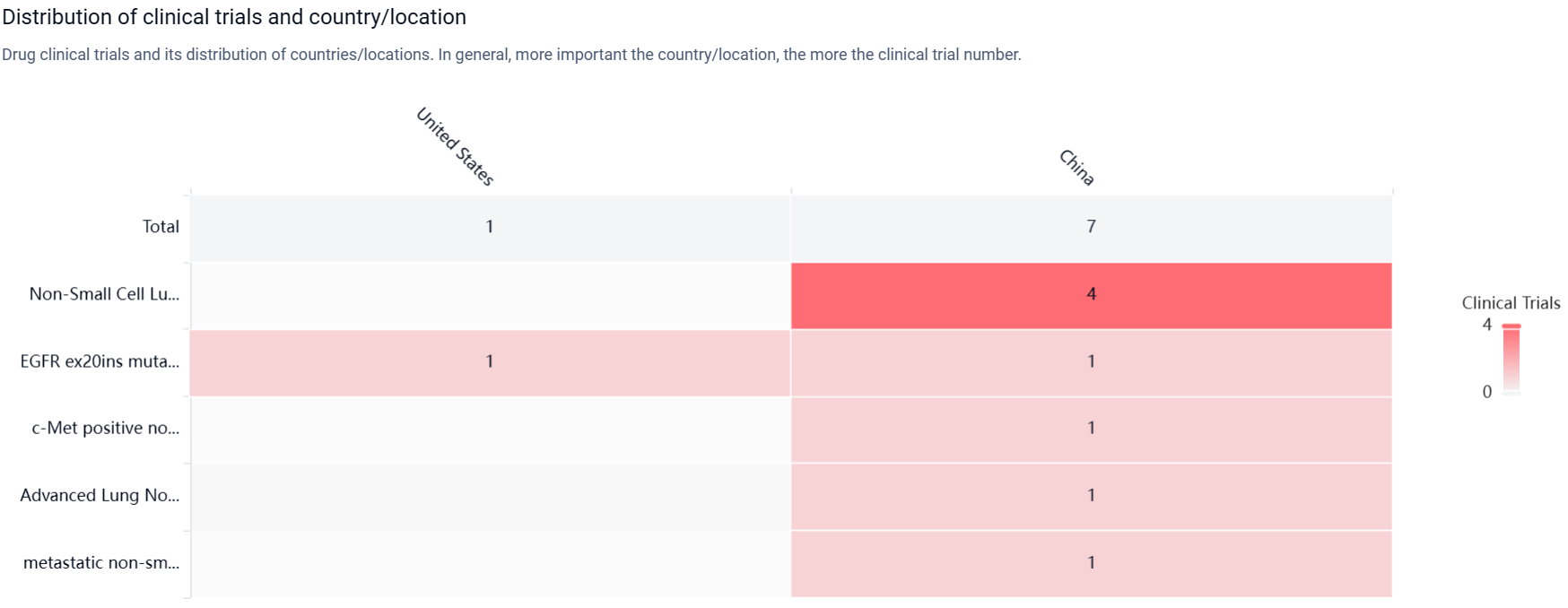
Detailed Clinical Result of PLB-1004
The multi-center, open-label, dose escalation and expansion study conducted entirely in China, to assess the safety, tolerability, pharmacokinetics, and anti-tumor effect of PLB1004.

In this study, PLB1004 was administered orally once per day, in patients with advanced non-small cell lung cancer. The primary objective of the study is to assess the safety profile of PLB1004 and determine the RP2D of the molecule.
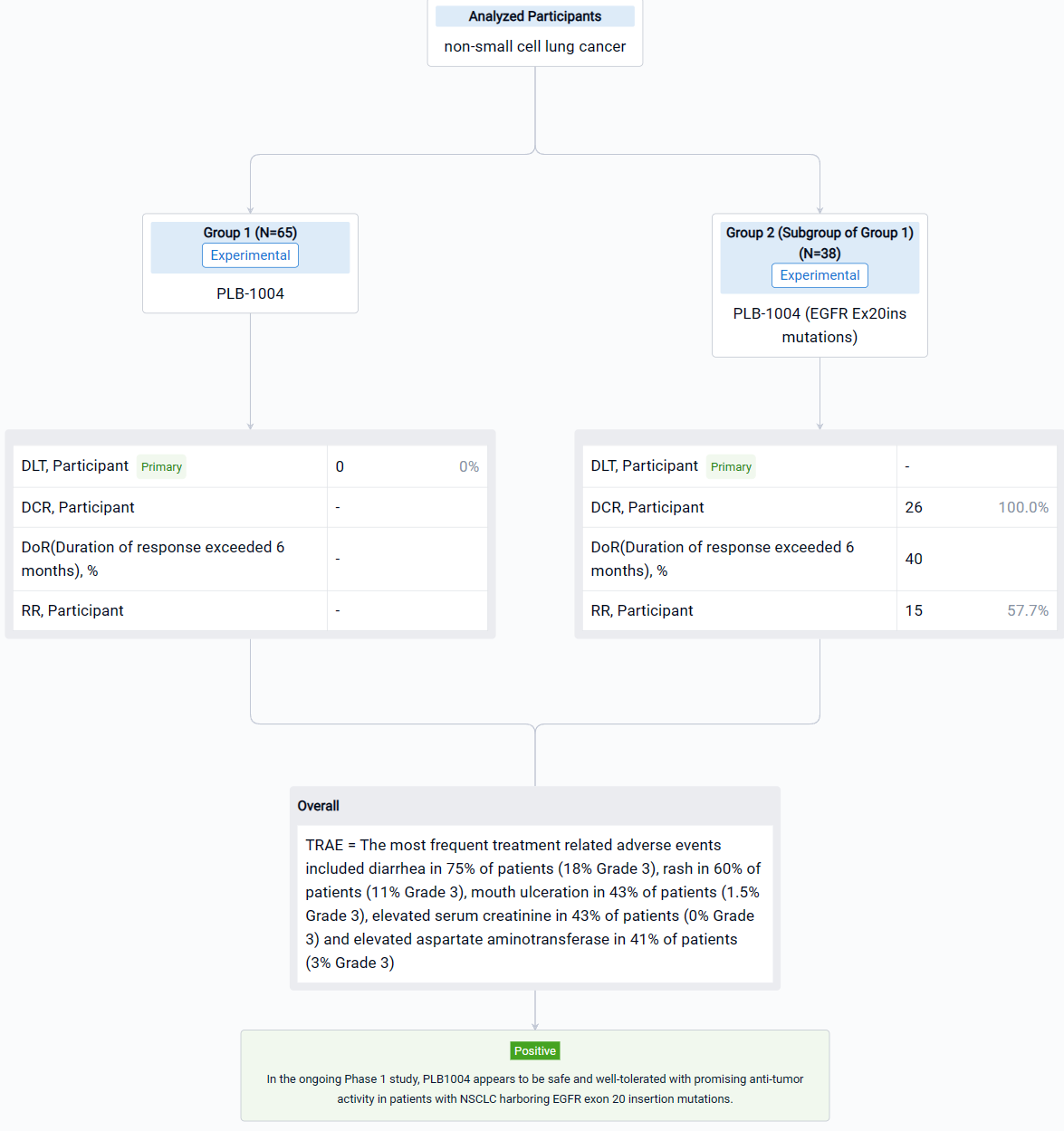
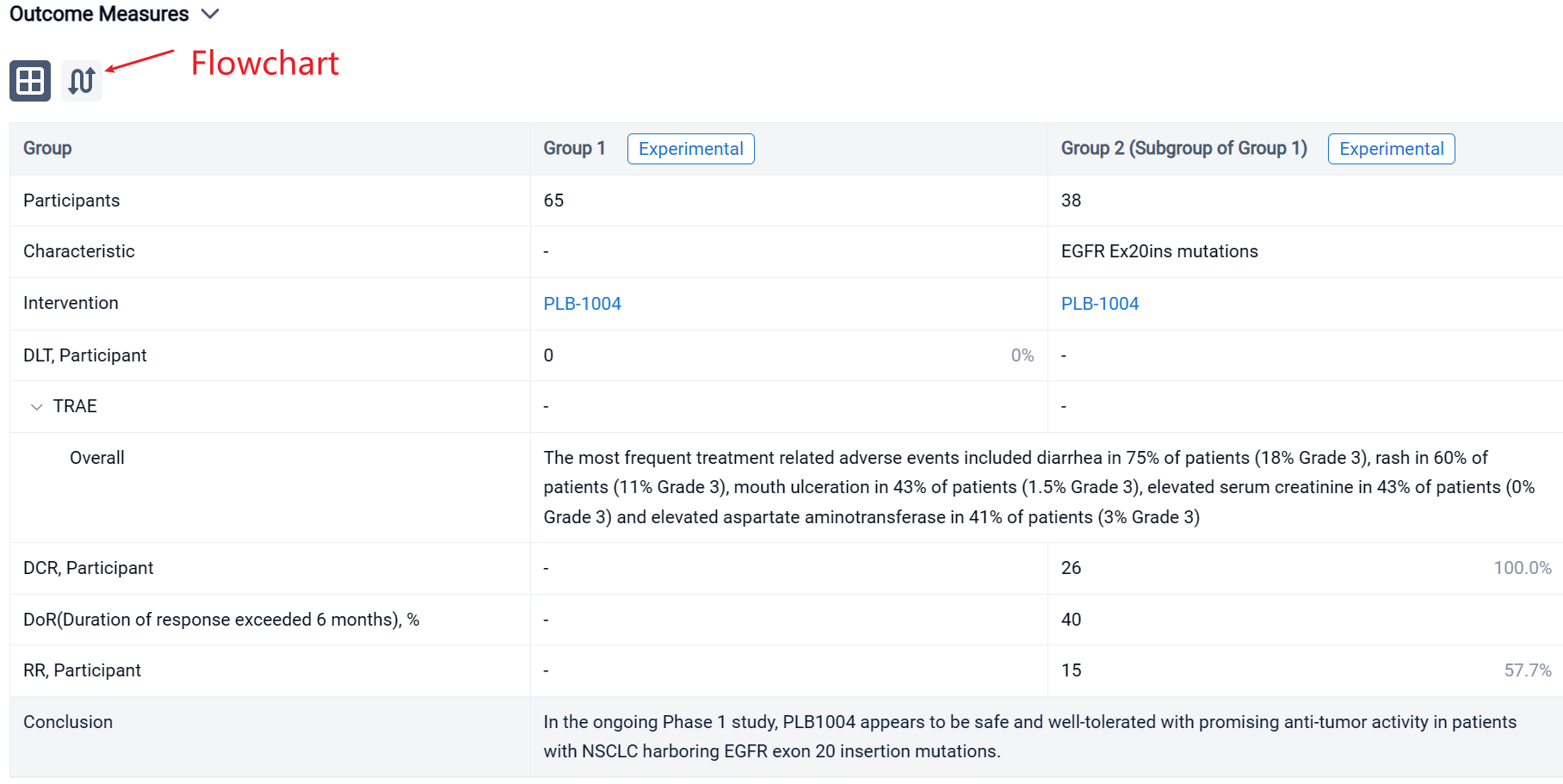
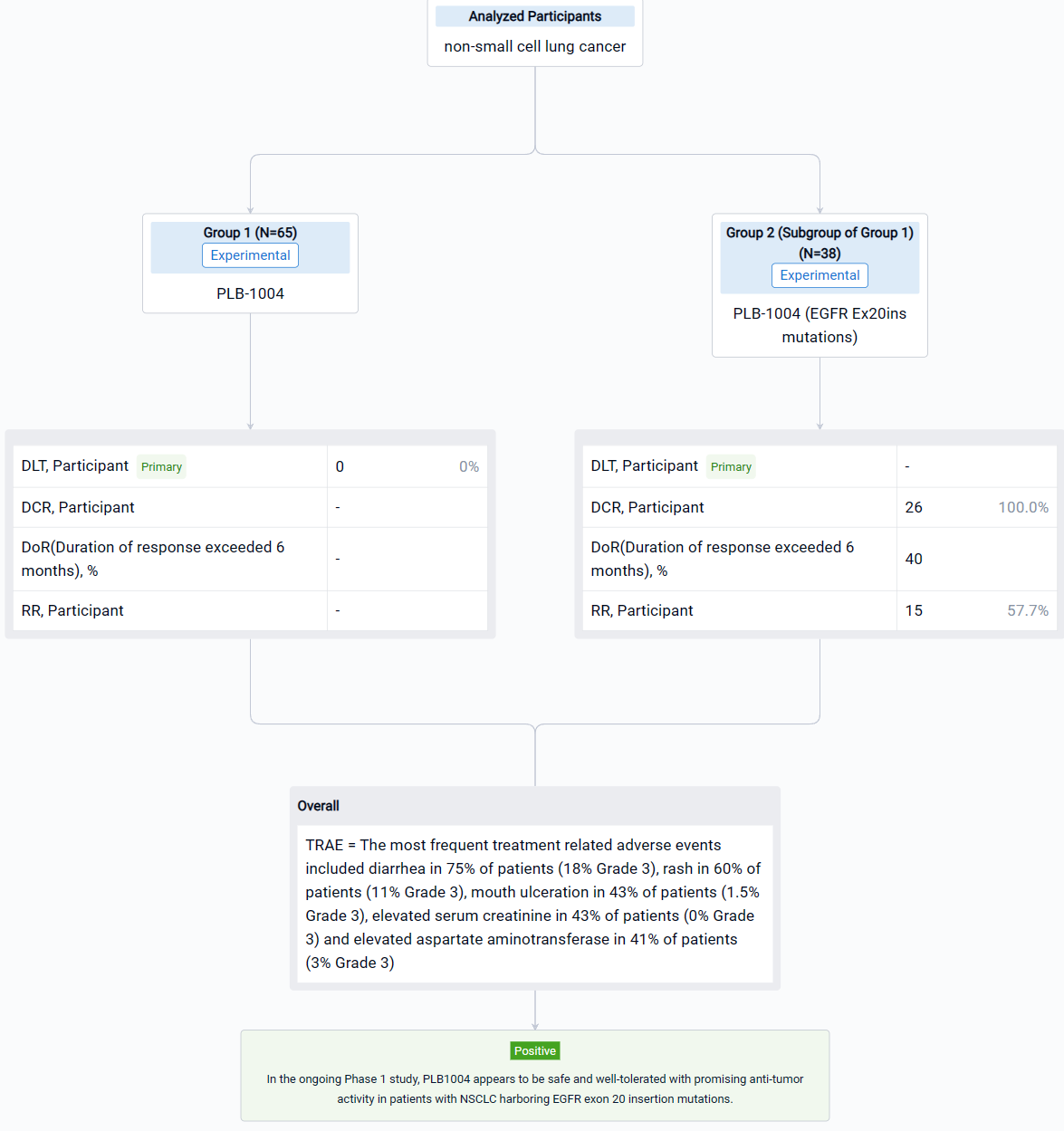
The result showed that Dose escalation ranged from a starting dose of 10 mg QD to a top dose of 480 mg QD in 11 cohorts of patients. Dose expansion is ongoing at two dose levels, 320 mg QD and 400 mg QD. At the cutoff date for this abstract, July 31, 2022, a total of 65 patients (32 in escalation and 33 in expansion) had received treatment with PLB1004. The median age of the patients is 58 years old (range 31 to 77). Most patients are women (60%) with adenocarcinoma (95%) and good performance status (ECOG 0-1 in 90%). Prior therapy for NSCLC included platinum-based chemotherapy in 54% of patients and TKI therapy in 58%. Of note 58% of patients had intra-cranial metastases at baseline. The most frequent treatment related adverse events included diarrhea in 75% of patients (18% Grade 3), rash in 60% of patients (11% Grade 3), mouth ulceration in 43% of patients (1.5% Grade 3), elevated serum creatinine in 43% of patients (0% Grade 3) and elevated aspartate aminotransferase in 41% of patients (3% Grade 3). The criteria for DLT were not reported at any dose level and thus an MTD was not determined during cycle 1 of drug administration. Beyond Cycle 1, at the highest dose levels, frequent dose interruptions and reductions due to toxicity were observed, and further dose escalation was not attempted above 480 mg QD. A more complete summary of safety data will be presented at the meeting. Across all dose groups, a total of 38 subjects had EGFR Ex20ins mutations, including 29 at doses ≥ 160 mg QD, among whom 26 completed at least 1 tumor assessment. In these 26 patients the confirmed response rate was 57.7% (15/26) and the disease control rate (DCR) was 100% (26/26). Duration of response exceeded 6 months in 40% of responders.
It can be concluded that in the ongoing Phase 1 study, PLB1004 appears to be safe and well-tolerated with promising anti-tumor activity in patients with NSCLC harboring EGFR exon 20 insertion mutations.
How to Easily View the Clinical Results Using Synapse Database?
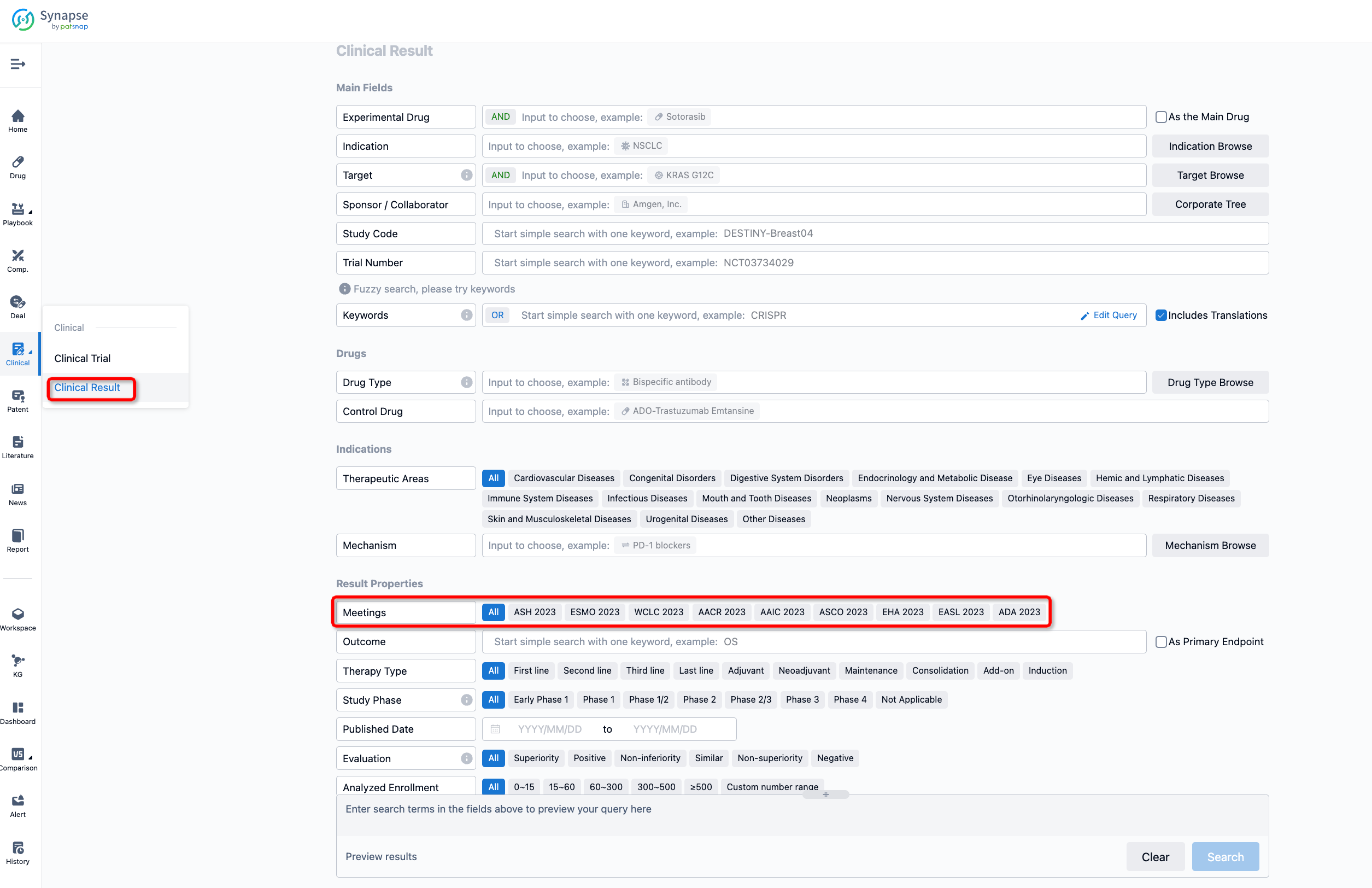
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
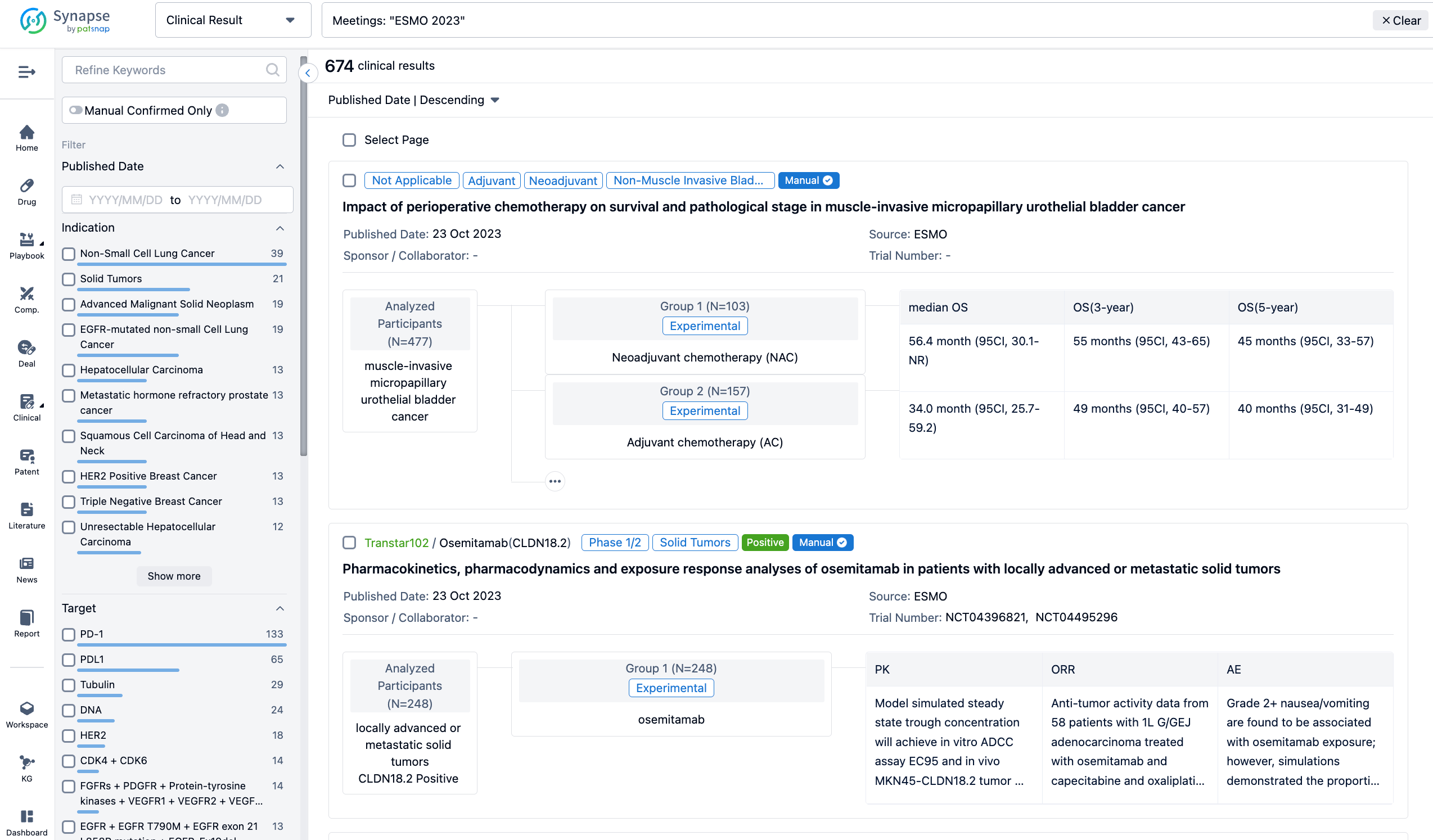
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
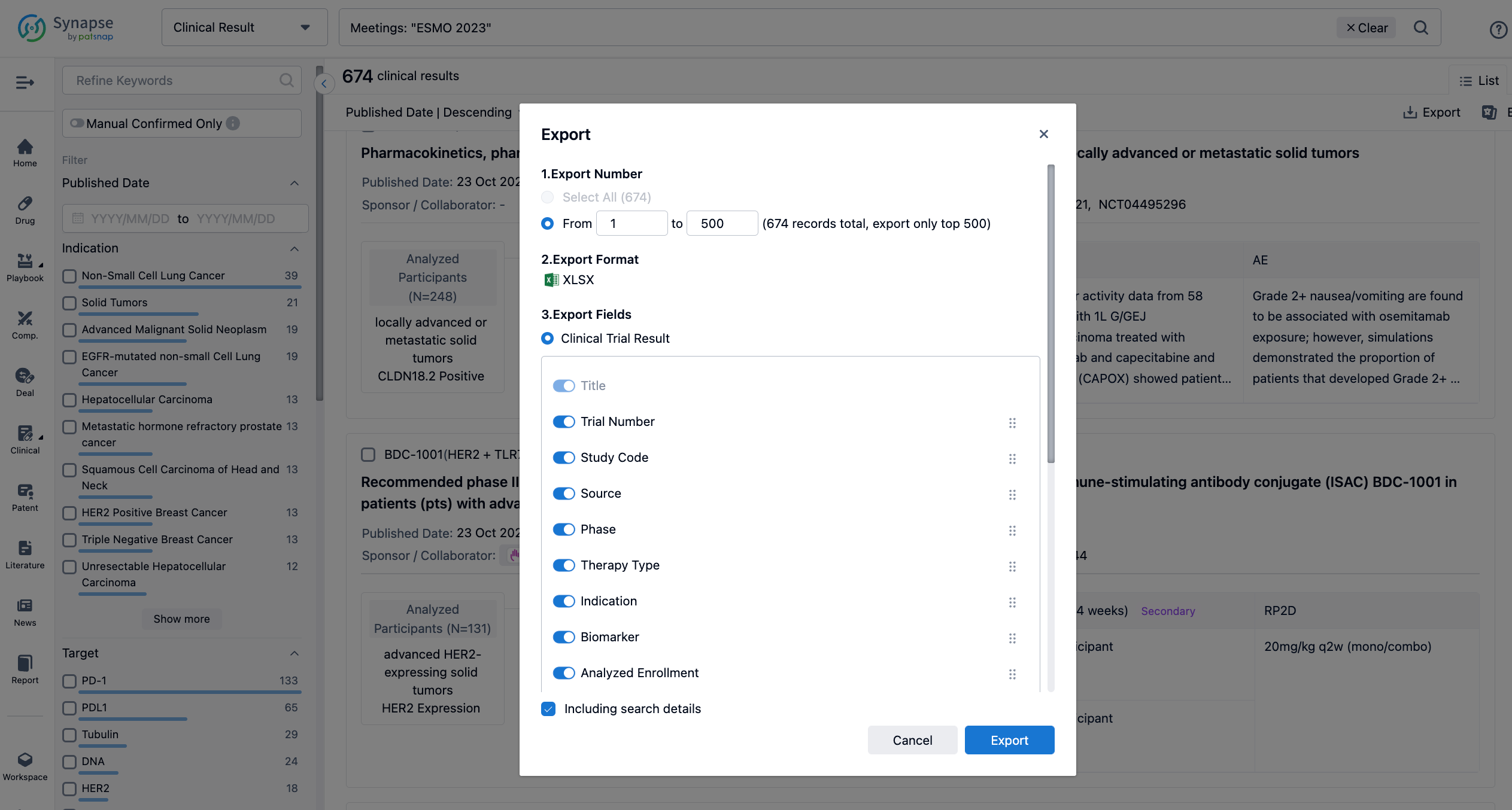
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!