Exploring the Latest PD-1 inhibitor Deal by ImmVirX: A Guide to Rapidly Accessing Transaction Insights
On February 21, 2024, Innovent Biologics and ImmVirX announced that they have entered into a clinical research and supply collaboration agreement to conduct clinical research on the combination therapy of sintilimab injection with the oncolytic virus candidate drug IVX037. ImmVirX is currently conducting a Phase I clinical study of IVX037 in Australia. A Phase Ib study is planned to start in mid-2024 in Australia to evaluate the anti-tumor activity and tolerability of IVX037 in combination with sintilimab (intravenous injection) in patients with advanced colorectal, ovarian, and gastric cancers, with a plan to enroll approximately 45 patients. According to the collaboration agreement, Innovent Biologics will supply sintilimab injection for this multicenter clinical trial.
About IVX037
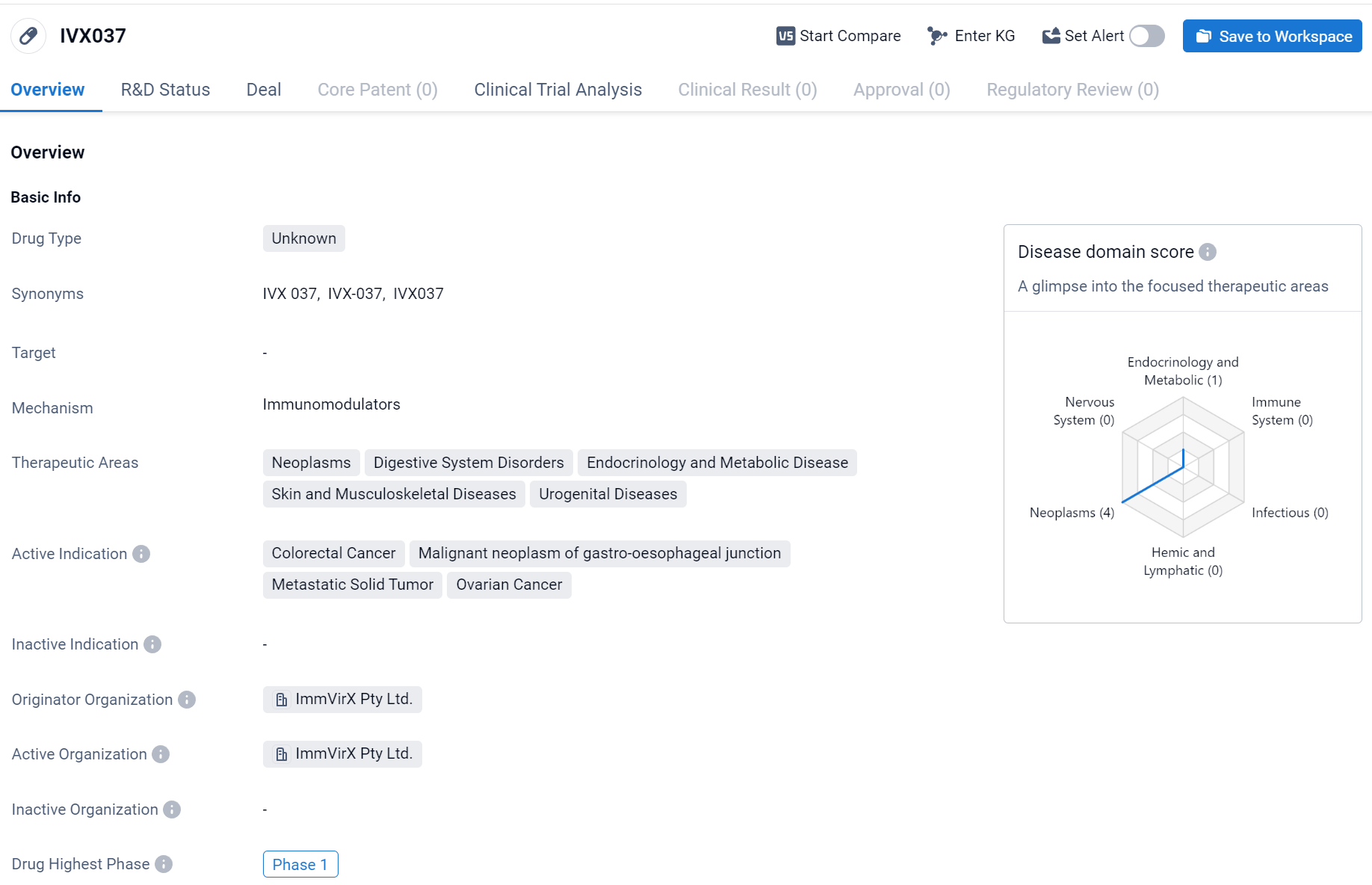
IVX037 is a drug in the field of biomedicine, with an unknown drug type. It is being developed for the treatment of various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. The drug is specifically indicated for the treatment of colorectal cancer, malignant neoplasm of gastro-oesophageal junction, metastatic solid tumor, and ovarian cancer. IVX037 is being developed by ImmVirX Pty Ltd., an originator organization in the pharmaceutical industry. The drug is currently in the highest phase of development, which is Phase 1. Click the image below to directly embark on the exploration journey with the IVX037!
The initial human Phase 1a portion of the trial for IVX037 was conducted in patients with advanced colorectal, gastric, or ovarian cancer. These three types of cancer are among the most common globally. The objective was to assess the safety, tolerability, and preliminary efficacy of IVX037. The preliminary results indicated that IVX037 was well-tolerated with multiple intratumoral injections as a monotherapy, showing early signs of inducing potentially beneficial inflammatory cytokines/chemokines, such as CXCL10. Additionally, anti-tumor activity was observed in injected lesions in some subjects.
About ImmVirX Pty
ImmVirX Pty Ltd. is a biomedicine organization based in Australia that was founded in 2019. The organization has developed drugs in various therapeutic areas, including Digestive System Disorders, Neoplasms, Skin and Musculoskeletal Diseases, Urogenital Diseases, and Endocrinology and Metabolic Disease. However, there is no data available on the most frequently developed targets. ImmVirX currently has one drug in Phase 1 of development.
The company's proprietary biopanning platform enables the development of RNA viruses targeted at specific receptor proteins, which are highly expressed across various cancer cell types. This allows for selective entry, replication, and destruction of tumor cells, while also potentially inducing beneficial changes within the tumor microenvironment, and prompting innate and adaptive immune responses against the cancer cells.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
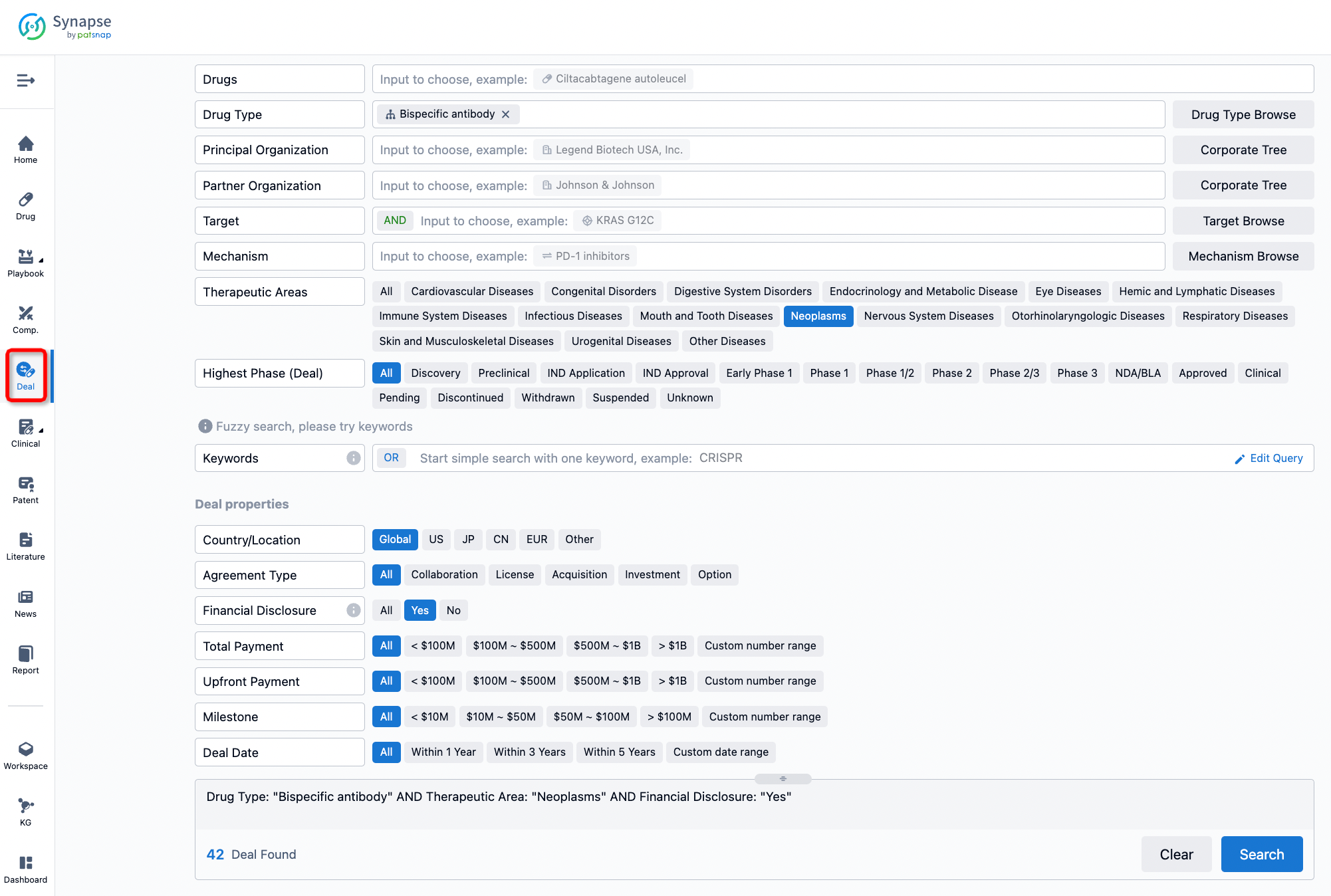
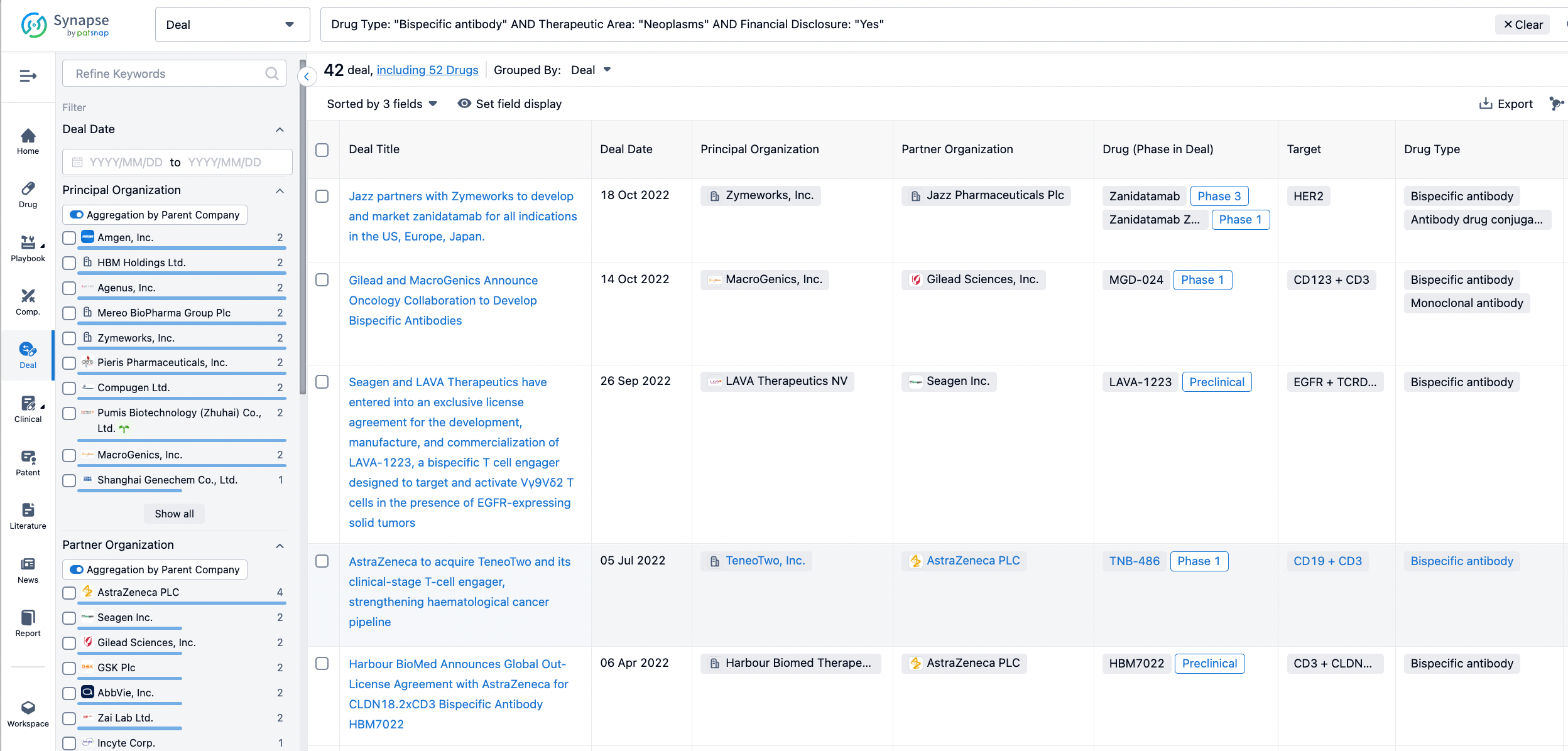
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
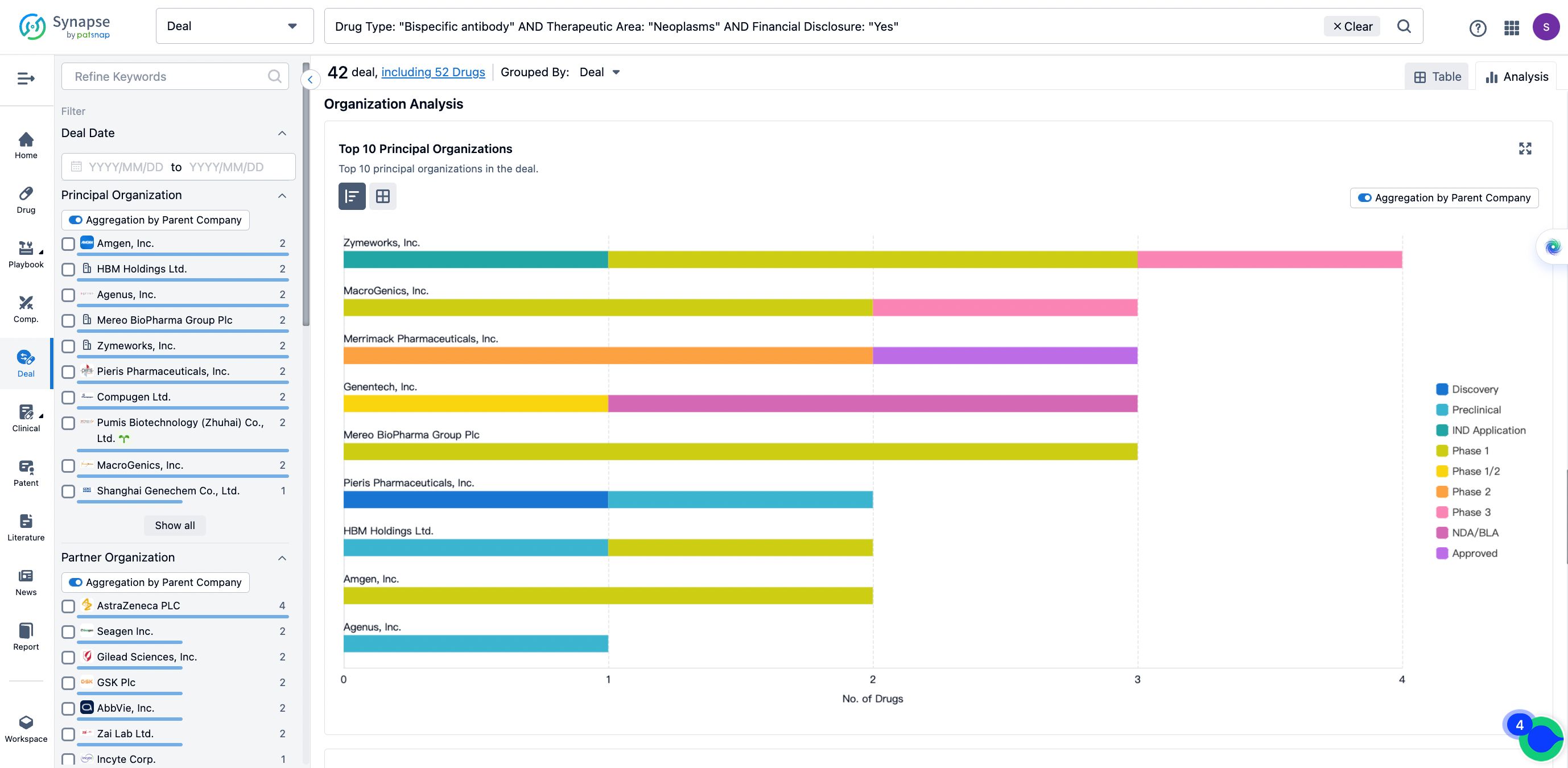
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
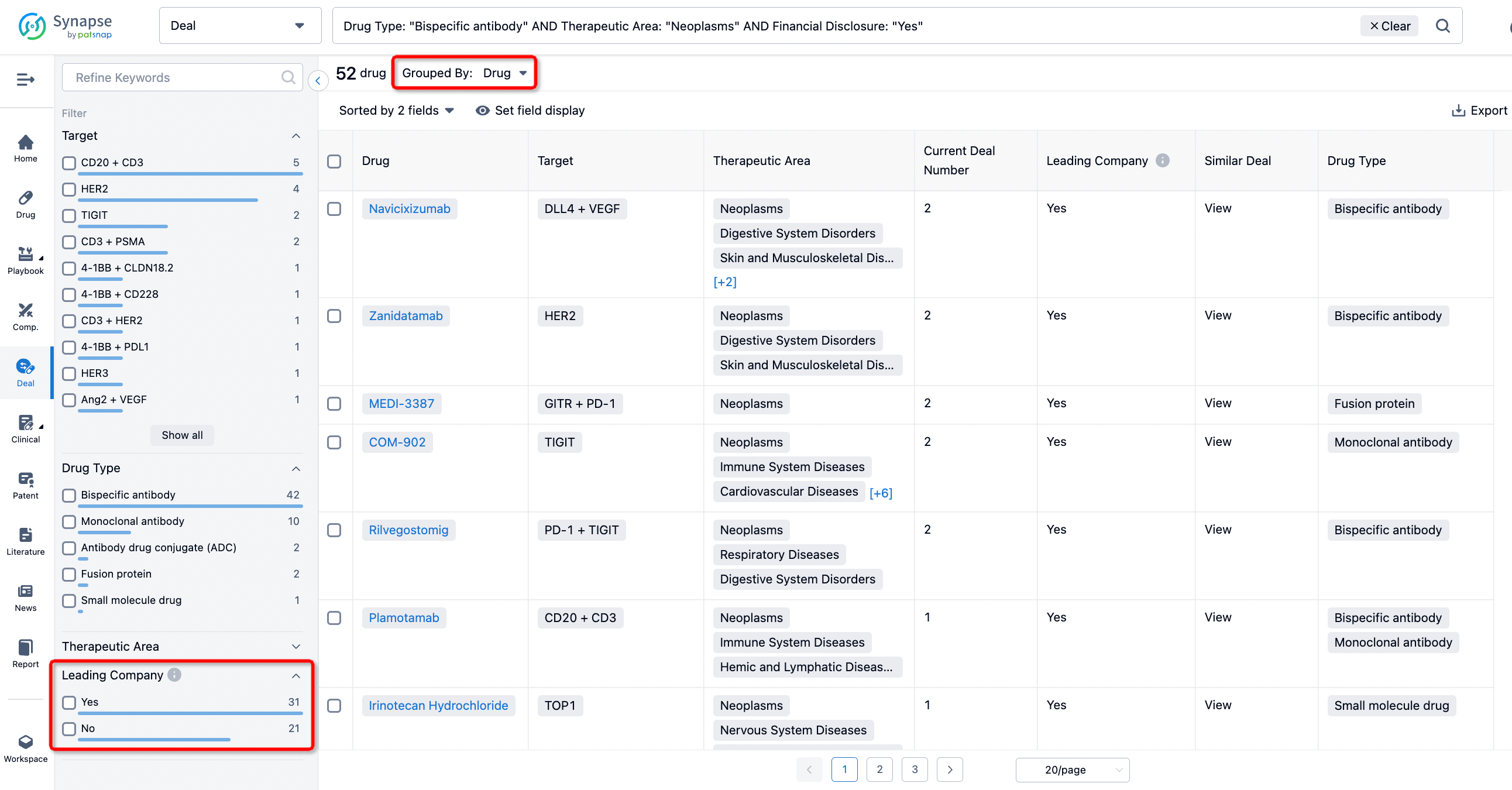
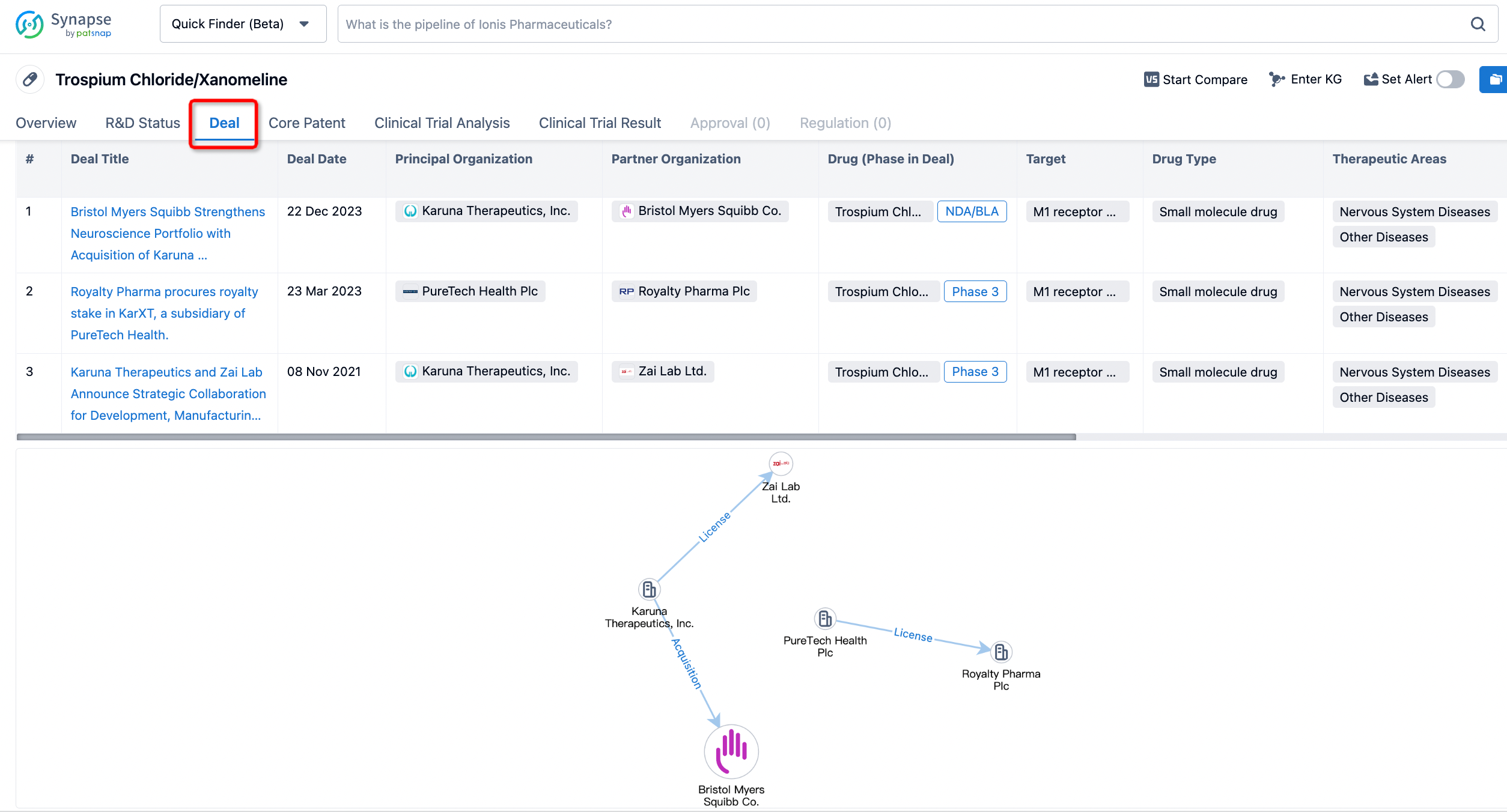
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
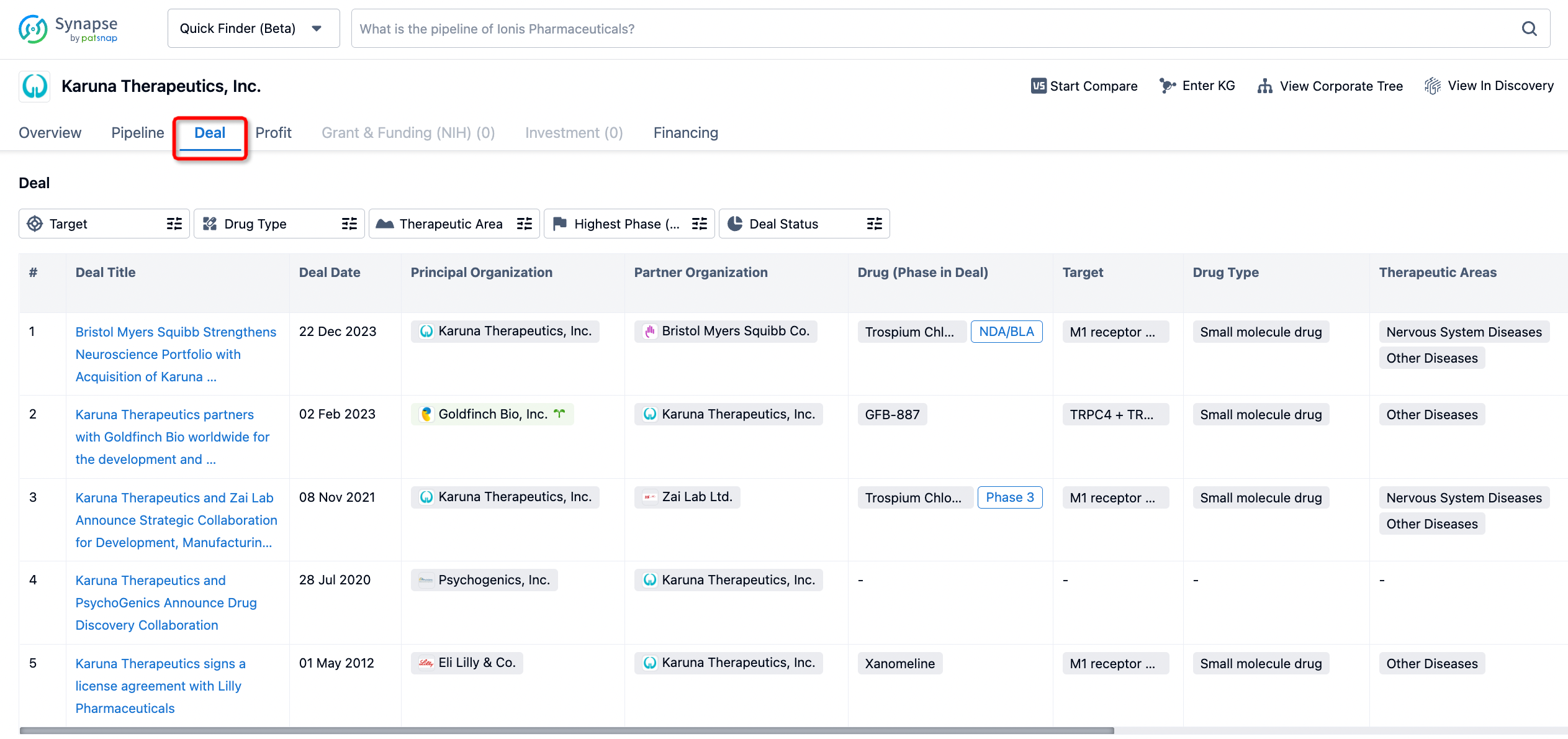
Click on the image below to embark on a brand new journey of drug discovery!