FDA Approves Alvotech-Teva's SIMLANDI®, New Biosimilar to Humira®
Alvotech, in partnership with Teva Pharmaceuticals, has declared the official sanction by the U.S. Food and Drug Administration of their product SIMLANDI (adalimumab-ryvk) for injectable use. This authorization recognizes SIMLANDI as a substitutable biosimilar for Humira. It is designated for the management of several adult conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel diseases such as Crohn's disease and ulcerative colitis. Further indications include treatments for adult plaque psoriasis, hidradenitis suppurativa, and uveitis, as well as juvenile idiopathic arthritis.
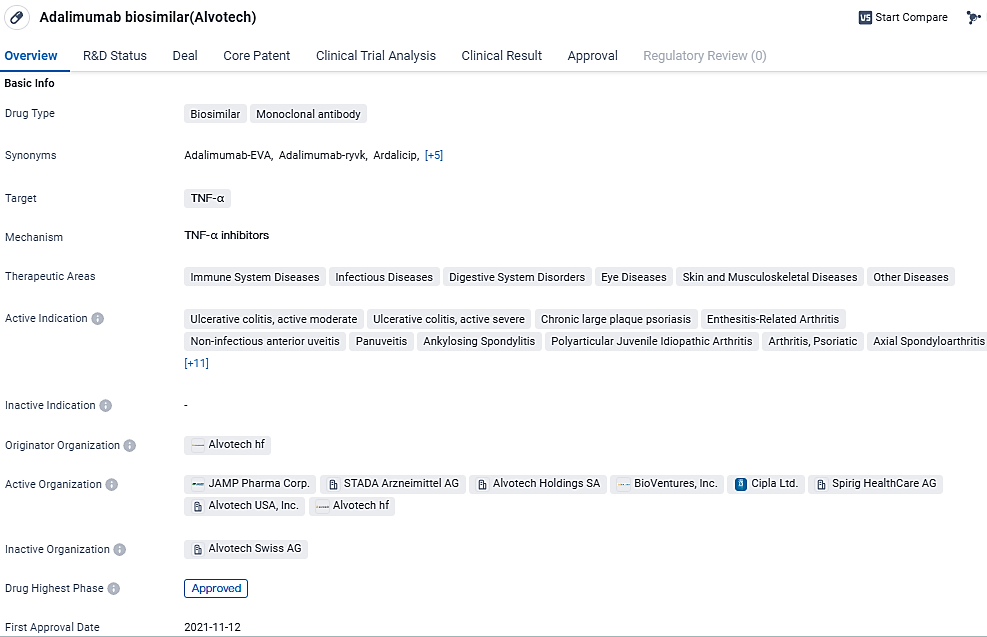
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the fiscal landscape of 2023, Humira emerged as a dominant force in the global pharmaceutical industry, achieving American sales nearing the $12.2 billion mark. Alvotech has designated Teva as the primary entity for the distribution of SIMLANDI within the United States market.
As the pioneering biosimilar to Humira exhibiting both a high concentration and lacking citrate, SIMLANDI has received interchangeability recognition by the FDA, and is set to receive an exclusivity period for the specific dosage of 40mg/0.4ml injection. Although the U.S. marketplace offers both lower and higher concentration versions of Humira biosimilars, the high-concentration version accounts for approximately 88 percent of all the adalimumab prescriptions within America.
Interchangeable biosimilars are distinctive in that they can be dispensed by pharmacists without a mandate to consult the original prescriber, analogous to how generic counterparts replace branded medications. As the sole interchangeable biosimilar to adalimumab with a high-concentration formula, SIMLANDI is poised to take Humira's place directly at the dispensing point, adhering to the regulations of individual state pharmacies.
Dr. Eric Hughes, who serves as Teva's Executive Vice President Global R&D and Chief Medical Officer, highlighted, “The sanction of SIMLANDI ushers in a new era as the first-of-its-kind high-concentration, citrate-free biosimilar to Humira with Interchangeable status. Teva, alongside Alvotech, celebrates this significant achievement as it aligns with our joint goal to propel seven biosimilars into the market, augmenting the breadth, affordability, and adoption of these alternatives in the States.”
August 2020 marked the inception of a collaborative effort between Alvotech and Teva, focusing on the sole promotion of five biosimilar candidates by Alvotech. Come August 2023, the synergy expanded to not only encompass two supplementary biosimilars but also included fresh variants of the products initially agreed upon.
Alvotech assumes responsibility for the development and production processes, while Teva is tasked with overseeing the introduction of these products into the American market. This strategy capitalizes on Teva's well-established market presence and promotional network. SIMLANDI, the first biosimilar approved as interchangeable under this concerted partnership, is anticipated by both Alvotech and Teva to make a prompt entrance into the U.S. marketplace, endorsed by its interchangeability merit.
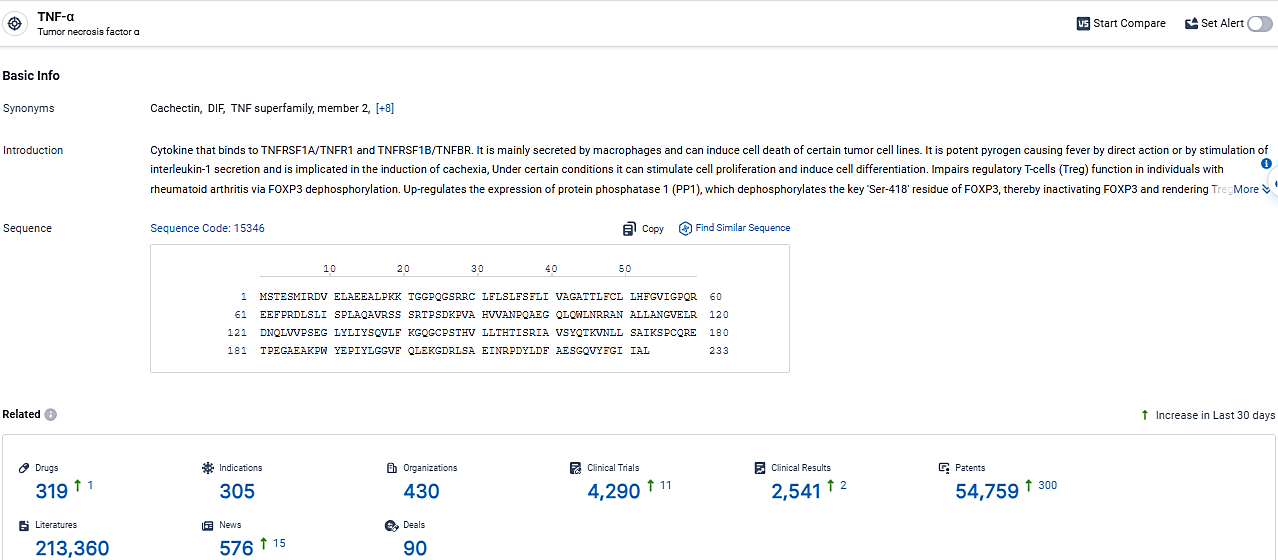
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 29, 2024, there are 319 investigational drugs for the TNF-α target, including 305 indications, 430 R&D institutions involved, with related clinical trials reaching 4290, and as many as 54759 patents.
SIMLANDI (adalimumab-ryvk) is a significant addition to the pharmaceutical industry, particularly in the field of biomedicine. The drug's monoclonal antibody nature allows it to specifically target TNF-α, a protein involved in inflammation and immune system regulation. As a biosimilar, it also contributes to cost savings in healthcare systems, making it a valuable addition to the pharmaceutical industry. Further research and clinical studies may explore additional therapeutic areas and indications for this drug, expanding its potential benefits to a wider patient population.