How to find the structure and classification of Ustekinumab?
Ustekinumab, also known by its brand name Stelara, is a groundbreaking drug targeting interleukin-12 (IL-12) and interleukin-23 (IL-23). Developed by Johnson & Johnson's Janssen Pharmaceuticals, Ustekinumab belongs to the category of monoclonal antibodies. It has revolutionized the treatment landscape for various inflammatory diseases, including plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. By inhibiting the activity of IL-12 and IL-23, Ustekinumab effectively reduces cytokine-mediated inflammatory responses, thereby alleviating symptoms and improving patients' quality of life.
Summary of Research Progress:
Ustekinumab has made significant strides in the field of immunology, particularly in managing chronic inflammatory conditions. The drug's mechanism of action involves blocking the interaction between IL-12 and IL-23 and their respective receptors, thereby reducing the production of pro-inflammatory cytokines and the subsequent immune response. This inhibition helps to alleviate symptoms and improve quality of life for patients suffering from these conditions.
Globally, Ustekinumab has been approved in numerous countries, including the United States, Europe, and Japan. It was first approved by the U.S. Food and Drug Administration (FDA) in 2009 for the treatment of moderate to severe plaque psoriasis. Since then, its indications have expanded to include psoriatic arthritis and Crohn's disease. The drug has also received approval in several other regions, solidifying its position in the global market.
The competitive landscape for Ustekinumab is robust, with several other biologics targeting similar pathways. Key competitors include adalimumab (Humira), etanercept (Enbrel), and secukinumab (Cosentyx). Despite this competition, Ustekinumab has maintained a strong market presence due to its unique mechanism of action and favorable safety profile. Clinical trials have consistently demonstrated its efficacy and safety, further supporting its use in clinical practice.The global competition pattern for Ustekinumab is intense, with several companies developing biosimilars, or copycat versions of the drug. In October 2023, the FDA approved Wezlana (ustekinumab-auub), developed by Amgen, as an interchangeable biosimilar to Stelara. This approval marks a significant milestone in the biosimilar industry, providing patients with more affordable and accessible treatment options. Other companies, such as Alvotech Swiss AG, are also developing biosimilars of Ustekinumab, with some already approved in certain markets.
Clinical research on Ustekinumab continues to expand, with ongoing studies exploring its potential in new indications and patient populations. For instance, Stelara has shown promising results in clinical trials for the treatment of ulcerative colitis, with patients experiencing sustained symptom relief for up to four years. These findings further solidify Ustekinumab's position as a leading treatment option for inflammatory diseases.
Type of Immunoglobulin of Ustekinumab
Ustekinumab is a human IgG1κ immunoglobulin. IgG1 is the most abundant subclass of immunoglobulins in human serum and is known for its ability to mediate various effector functions, including complement activation and antibody-dependent cell-mediated cytotoxicity (ADCC). The κ light chain is one of the two types of light chains found in immunoglobulins, the other being λ. The κ light chain is more common and provides stability and specificity to the antibody structure.
Light and Heavy Chains and Structural Characteristics of Ustekinumab
Ustekinumab's structure consists of two heavy chains and two light chains, each with specific structural and functional characteristics. The heavy chains, which contain about 450 amino acid residues, are divided into two subclasses: IgG1 and IgGκ. The light chains are of the κ type. Both heavy and light chains contain variable and constant regions. The variable regions determine the antibody's specificity, while the constant regions maintain the antibody's stability and structure.
The heavy and light chains of Ustekinumab are connected by disulfide bonds, ensuring the antibody's overall structural stability. The Fab region of the antibody, located at the N-terminal end of each heavy and light chain, is responsible for antigen binding. This region contains antigen-binding sites that specifically bind to the p40 subunit of IL-12 and IL-23, thereby inhibiting their activity.
The heavy chain of Ustekinumab is a human IgG1 molecule, which consists of four domains: variable (VH), constant 1 (CH1), constant 2 (CH2), and constant 3 (CH3). The VH domain is responsible for antigen binding, while the CH1 domain forms part of the Fc region, which is crucial for the antibody's effector functions. The CH2 and CH3 domains are involved in the interaction with Fc receptors and complement proteins, respectively.
The Fc region of Ustekinumab, located at the C-terminal end of the heavy chains, plays a crucial role in immune effector functions. It binds to Fc receptors on immune cells, such as macrophages and natural killer cells, activating these cells and regulating the immune response. The Fc region also contributes to the antibody's stability and solubility, ensuring its effectiveness in vivo.
The light chain of Ustekinumab is a κ chain, which also consists of two domains: variable (VL) and constant (CL). The VL domain pairs with the VH domain to form the antigen-binding site, ensuring high specificity and affinity for the target antigen. The CL domain, like the CH1 domain, contributes to the stability and function of the antibody.
The unique structural characteristics of Ustekinumab, including its specific antigen-binding sites and Fc region, make it an effective and safe treatment option for inflammatory diseases. By inhibiting the activity of IL-12 and IL-23, Ustekinumab reduces inflammatory responses and alleviates symptoms, improving patients' quality of life.
Summary and Prospect
In summary, Ustekinumab is a human monoclonal antibody that targets the p40 subunit of IL-12 and IL-23, making it a valuable therapeutic option for managing chronic inflammatory conditions such as moderate to severe plaque psoriasis, psoriatic arthritis, and Crohn's disease. Its unique mechanism of action, coupled with a favorable safety profile, has led to its widespread adoption and approval in multiple countries. As a human IgG1κ immunoglobulin, Ustekinumab exhibits well-defined structural characteristics that contribute to its high specificity and efficacy. The heavy and light chains, with their respective variable and constant domains, ensure precise antigen binding and robust effector functions, making Ustekinumab a promising and effective treatment for a range of autoimmune disorders. Future research and development efforts will likely focus on optimizing its delivery methods, exploring new indications, and enhancing its therapeutic benefits for patients.
How to find the structure and classification of antibody drugs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all antibody drugs.
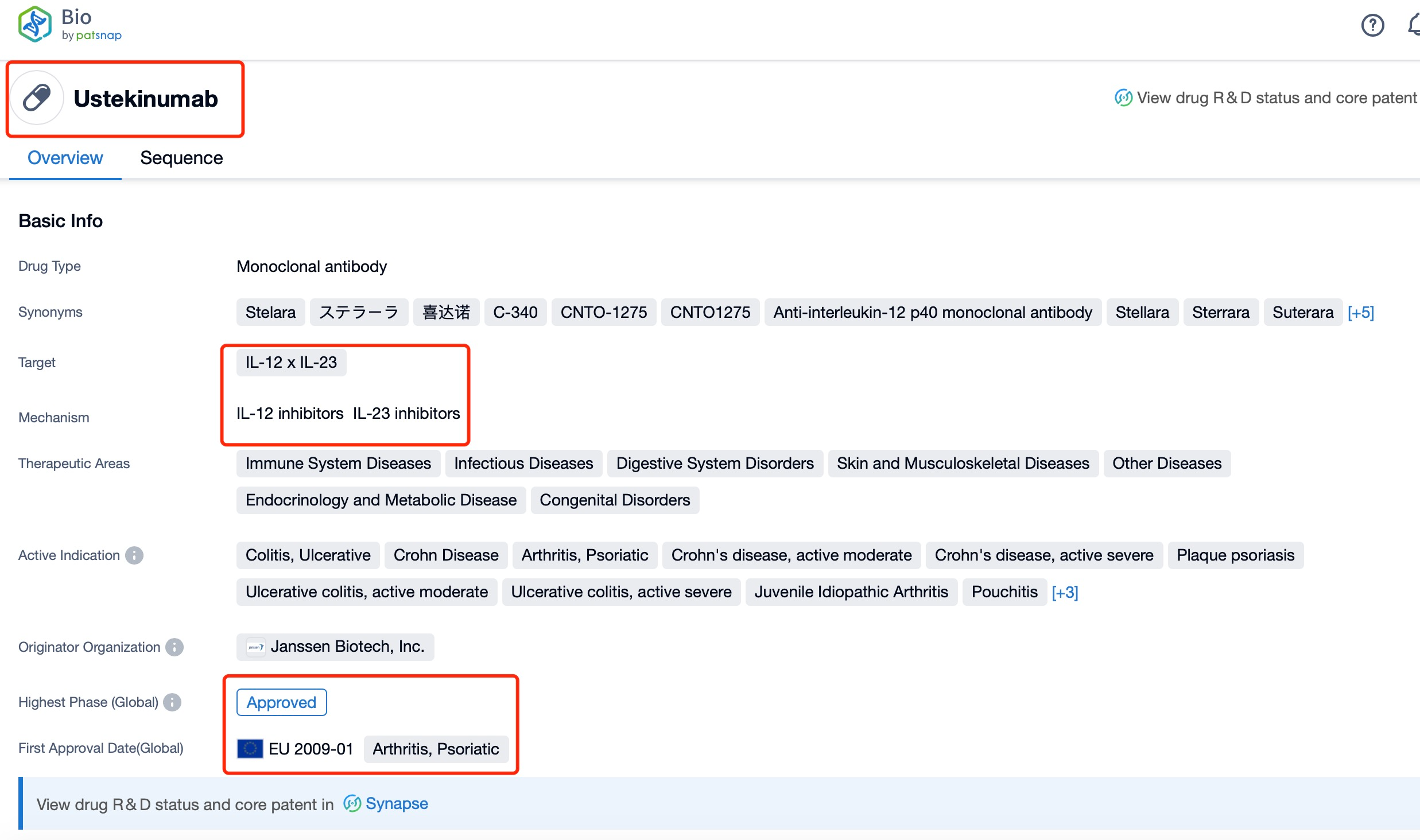
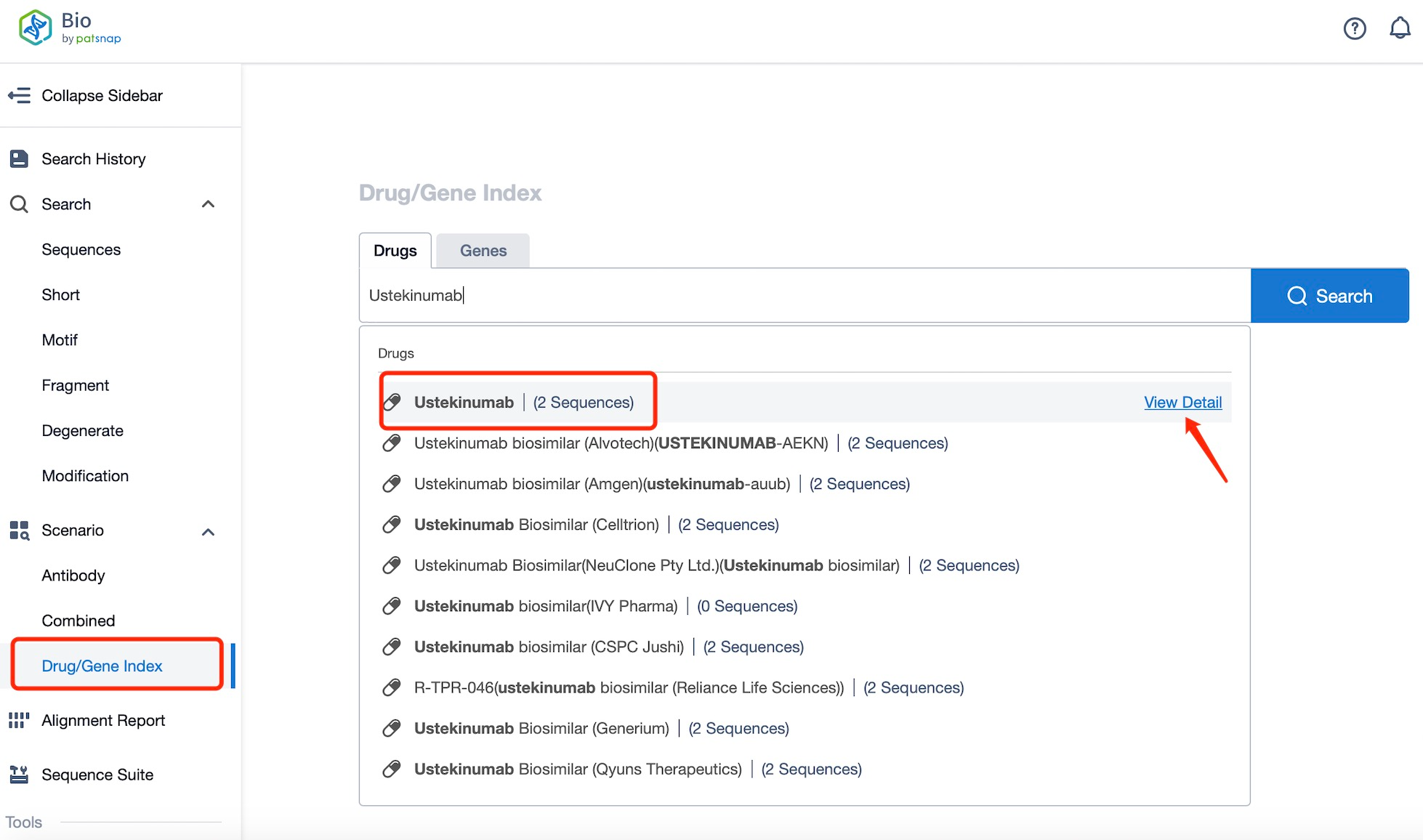
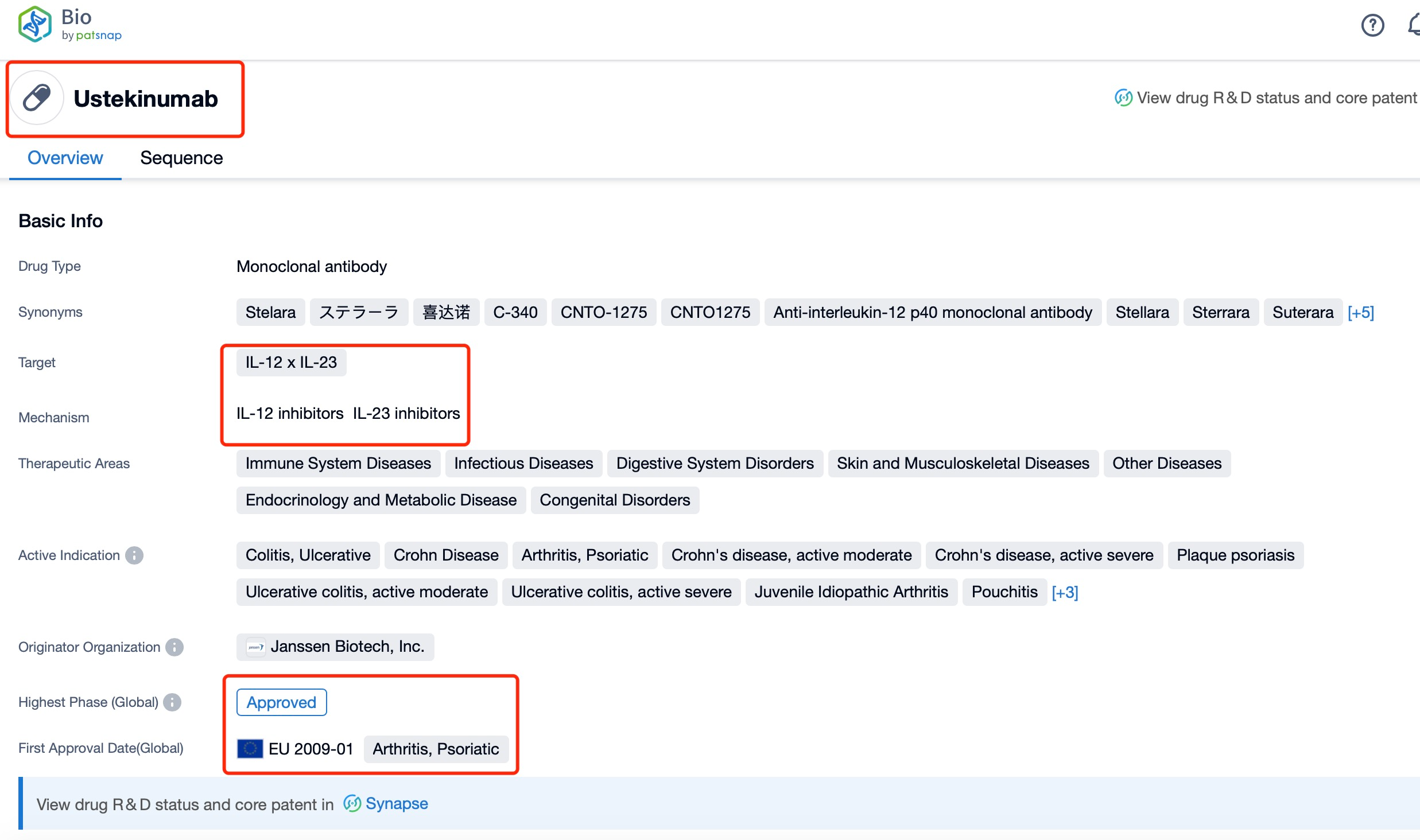
Taking Ustekinumab as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Ustekinumab ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Ustekinumab.
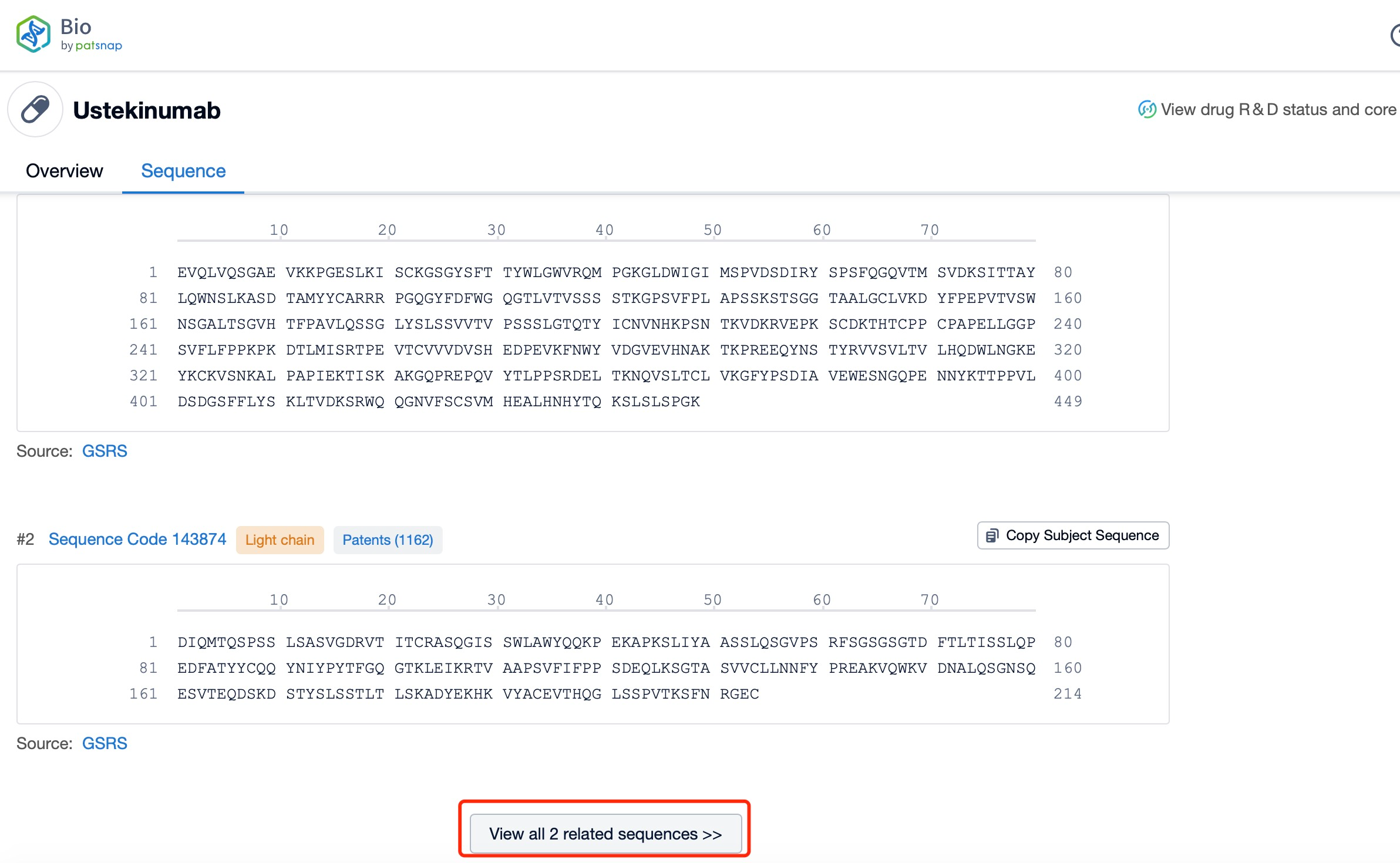
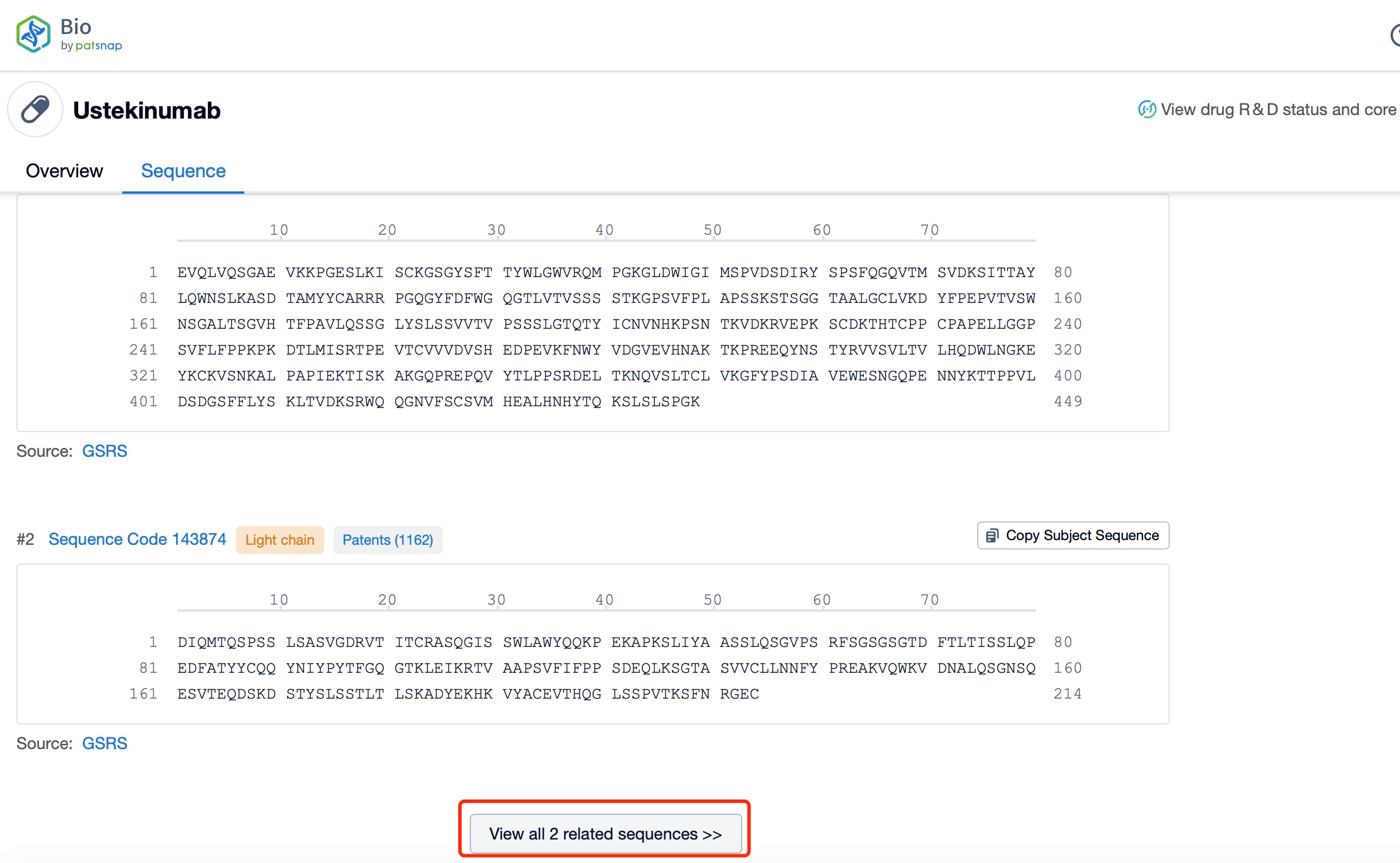
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
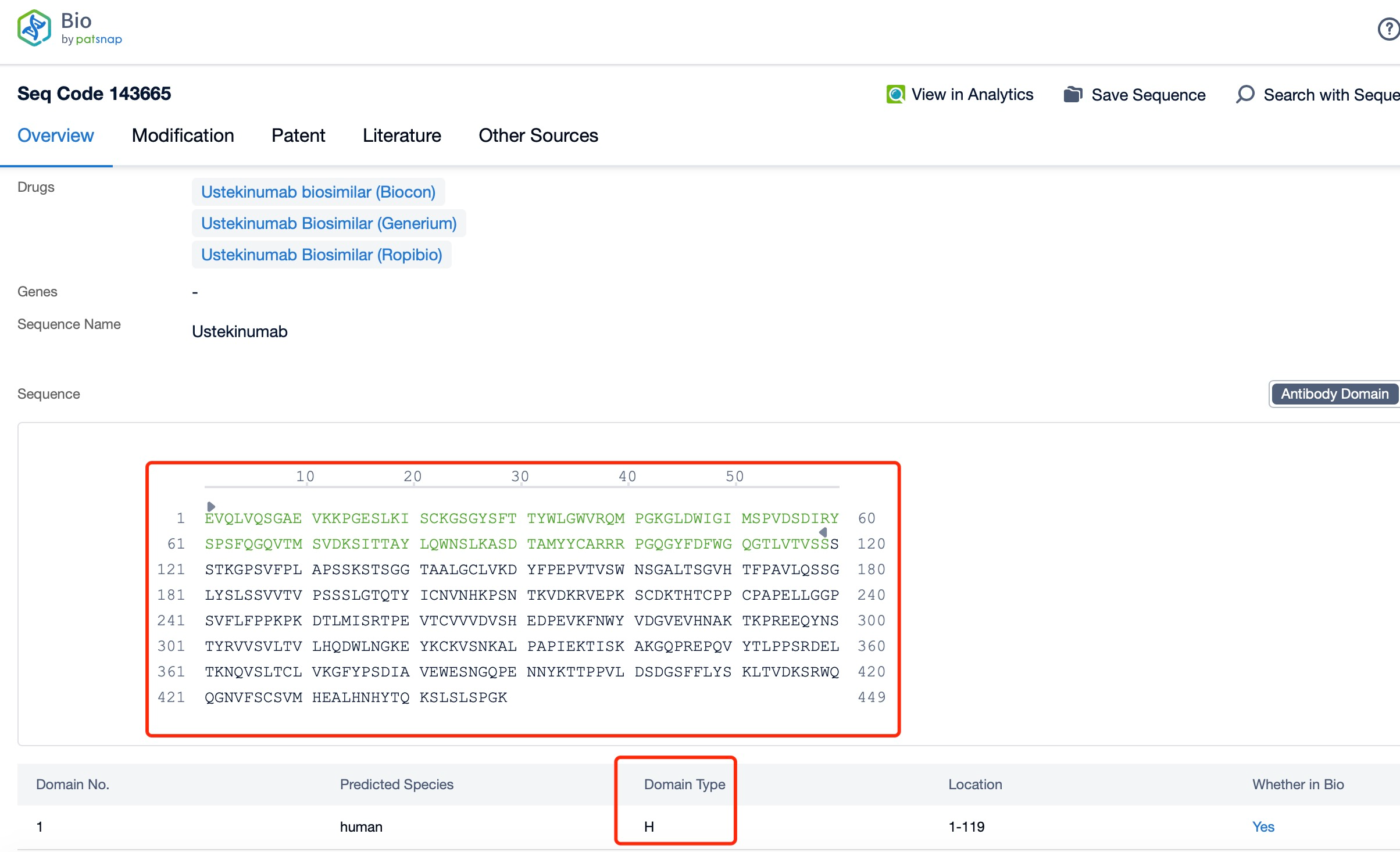
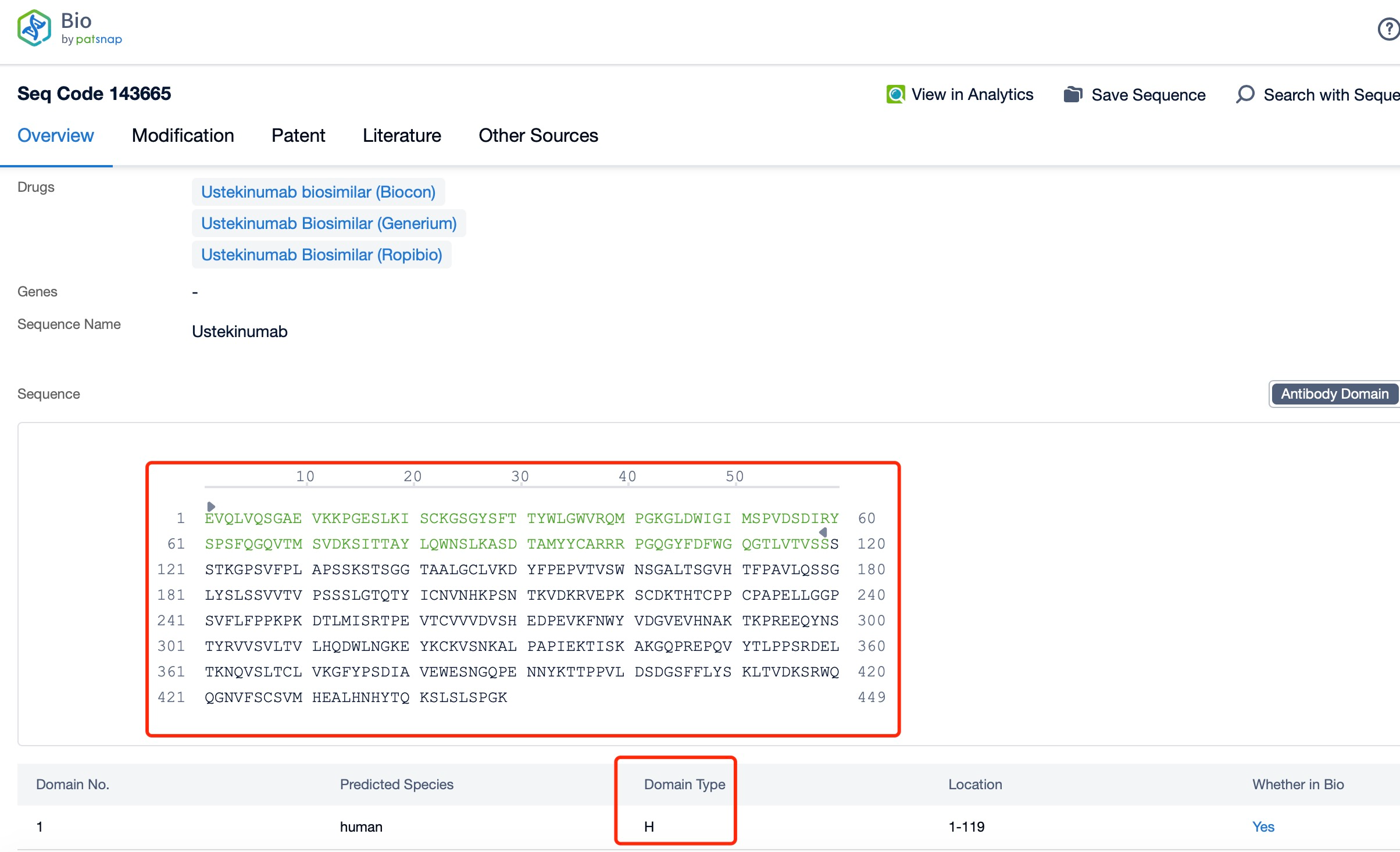
Clicking on the sequence name will provide you with all the basic information of that sequence. Here, you can easily query the sequence and action of the light and light chains of antibodies.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.