Lilly's tirzepatide lowered the chance of worsening heart failure events by 38% in adults who have HFpEF and obesity
Eli Lilly and Company (NYSE: LLY) revealed comprehensive findings from the SUMMIT Phase 3 trial, which demonstrated that tirzepatide considerably lowered the likelihood of adverse heart failure events in adults suffering from heart failure with preserved ejection fraction (HFpEF) alongside obesity. Individuals receiving tirzepatide showed significant enhancements in heart failure symptoms as well as physical restrictions. These findings were published in The New England Journal of Medicine concurrently with a presentation at the American Heart Association (AHA) Scientific Sessions 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Both primary objectives were successfully achieved. Tirzepatide resulted in a 38% lower risk of heart failure outcomes, evaluated as a composite measure, in comparison to the placebo group. The risk of being hospitalized due to heart failure was diminished by 56%. Furthermore, participants receiving tirzepatide experienced an almost 25-point enhancement in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), a tool for assessing symptoms and physical restrictions related to heart failure, while the placebo cohort improved by 15 points.
“Numerous studies indicate that obesity significantly contributes to the onset and severity of heart failure with preserved ejection fraction by exacerbating systemic and myocardial inflammation,” stated Milton Packer, M.D., a distinguished cardiovascular science scholar at Baylor University Medical Center in Dallas and visiting professor at Imperial College London (chair of the steering committee). “The SUMMIT trial offers valuable insights into how healthcare professionals can positively influence the clinical trajectory and quality of life for patients dealing with HFpEF and obesity.”
All primary secondary endpoints were also achieved, with subjects receiving tirzepatide showing enhanced exercise performance, walking around 30 meters further in a six-minute test compared to the placebo group (38.2 meters vs. 7.9 meters). In addition, those on tirzepatide experienced an average weight loss of 15.7%, while the placebo group recorded only a 2.2% reduction. Tirzepatide also led to a significant 43.4% decrease in high-sensitivity C-reactive protein (hsCRP), a critical indicator of systemic inflammation, compared to a modest 3.5% decrease in the placebo group.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
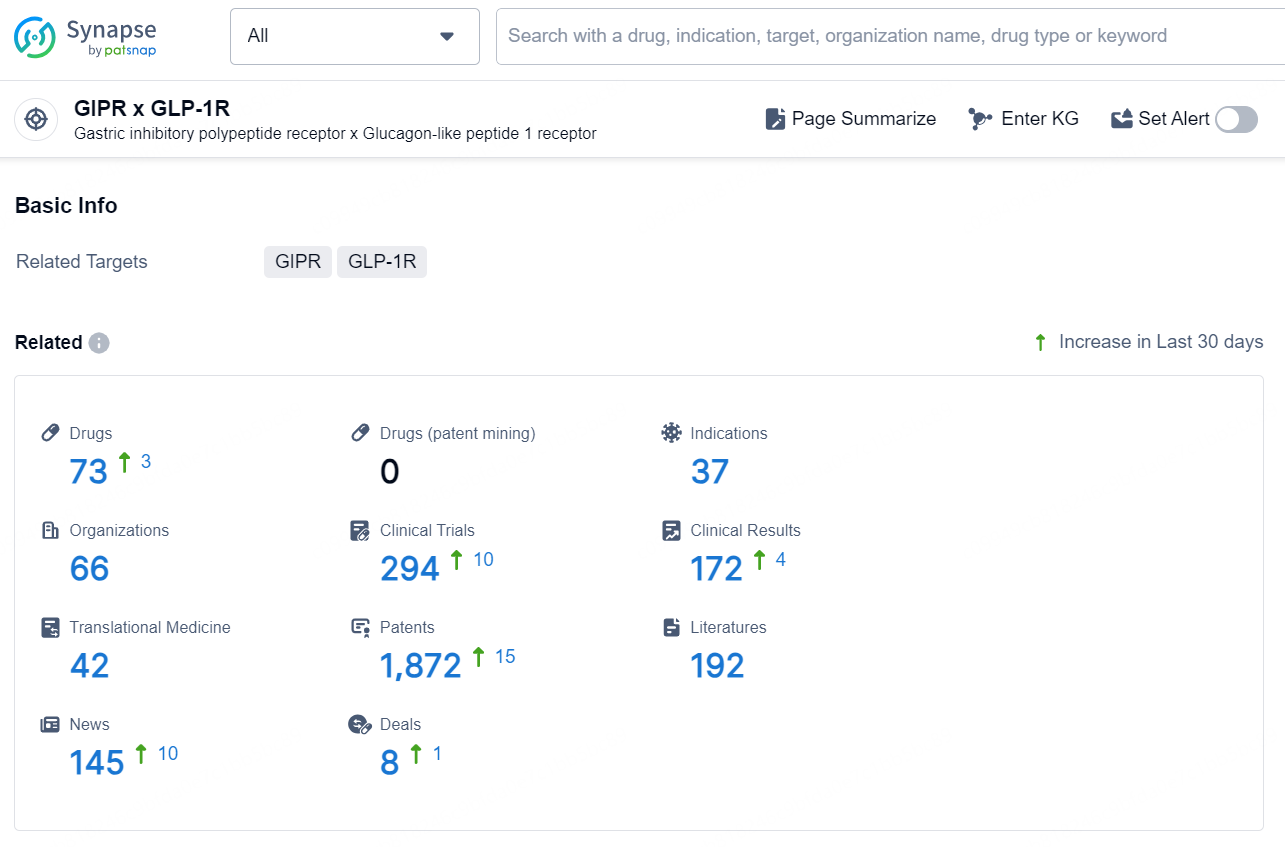
According to the data provided by the Synapse Database, As of November 20, 2024, there are 73 investigational drug for the GIPR x GLP-1R target, including 37 indications, 66 R&D institutions involved, with related clinical trials reaching 294, and as many as 1872 patents.
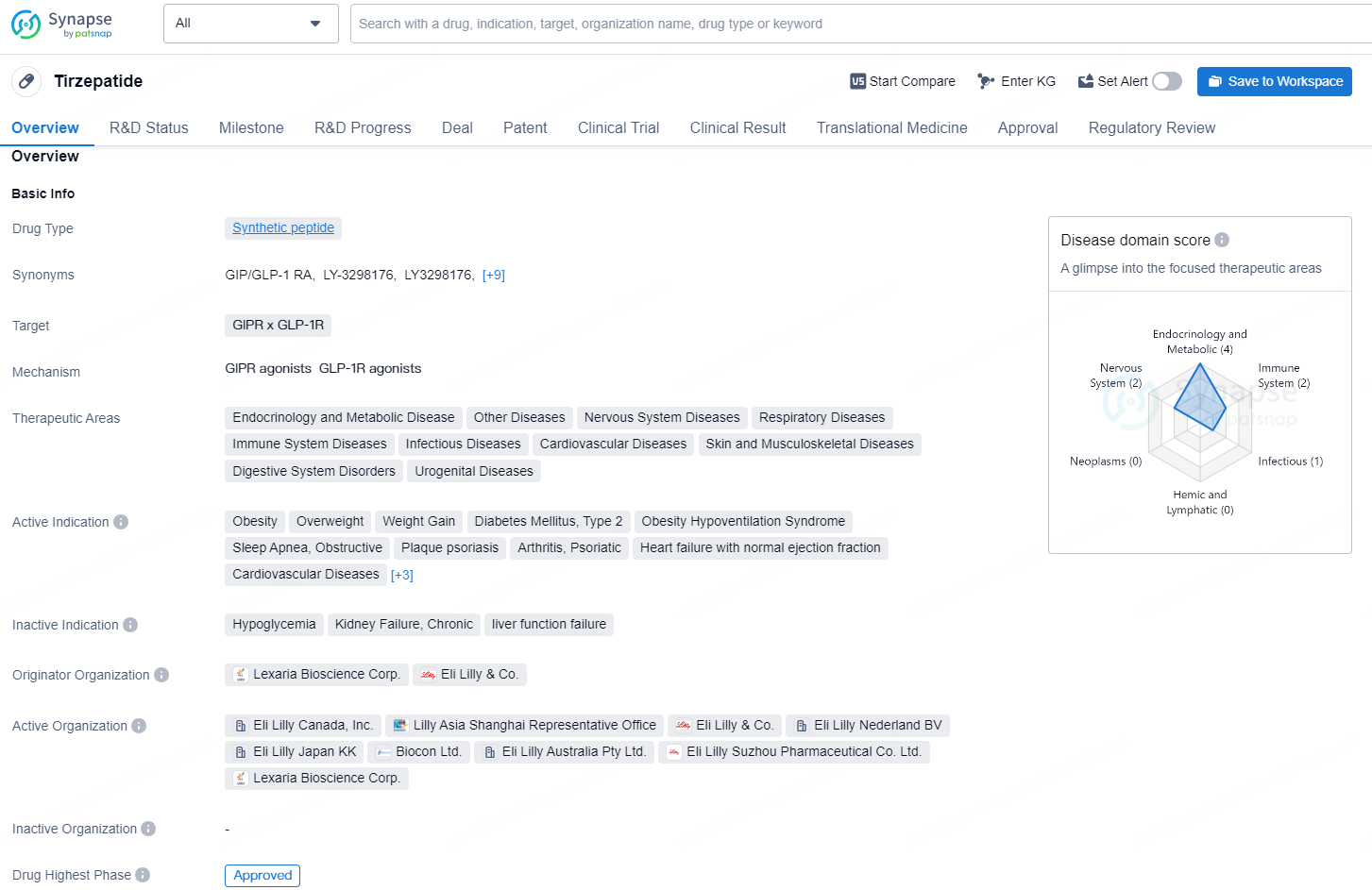
Tirzepatide is a synthetic peptide drug that targets GIPR x GLP-1R, with a wide range of therapeutic areas including endocrinology and metabolic disease, nervous system diseases, respiratory diseases, immune system diseases, infectious diseases, cardiovascular diseases, and others.