Favorable Phase 1b Results for Sorriso's SOR102 in Ulcerative Colitis Treatment
Sorriso Pharmaceuticals, a biopharmaceutical firm focused on creating innovative orally administered antibodies for immune-mediated conditions, has announced encouraging findings from its Phase 1b clinical trial of SOR102. This oral dual-action biologic targets TNFα and IL-23 in individuals diagnosed with ulcerative colitis (UC). The trial achieved its primary goals, highlighting a positive safety and tolerability profile. Furthermore, significant exploratory efficacy endpoints indicated the promising effectiveness of SOR102 across various clinical measures. This marks the first successful evidence of an orally administered antibody providing clinical benefits.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Our Phase 1b findings with SOR102 represent a pivotal achievement for Sorriso Pharmaceuticals and the domain of autoimmune treatments,” stated Ciara Kennedy, the Chief Executive Officer of Sorriso Pharmaceuticals. “We believe SOR102, an innovative oral biologic that targets TNFα and IL-23—both well-recognized pathways in inflammatory bowel disease—has the potential to be transformative. The clinical findings for SOR102 present compelling evidence for its capability to offer an effective and convenient oral treatment option for those suffering from ulcerative colitis. We are eager to proceed to Phase 2 development for SOR102."
The randomized, double-blind, placebo-controlled study included 22 participants at two different sites. Subjects were randomly assigned to receive either one of two SOR102 dosages or a placebo over a duration of six weeks. The trial evaluated safety, tolerability, pharmacokinetics, pharmacodynamics, immunogenicity, and initial efficacy. Among the patients who completed the trial, those receiving the higher dosage of SOR102 showed statistically significant improvements over placebo across several clinical indicators, including responses measured by the Mayo Score, modified Mayo Score, symptomatic remission, and average reductions from baseline in Mayo Score, modified Mayo Score, and UC-100 score.
Given these outcomes, Sorriso Pharmaceuticals intends to launch a Phase 2 clinical trial in 2025 to further investigate SOR102 in a broader patient cohort. "Ulcerative colitis presents ongoing challenges, yet we have observed promising clinical remission rates from dual inhibition of TNFα and IL-23 through systemically administered antibodies," remarked Carlos Sattler, the Chief Medical Officer of Sorriso Pharmaceuticals. "The improvements seen after just six weeks of treatment are competitive with existing therapies and highlight SOR102’s potential to reshape treatment strategies by addressing these two pivotal inflammatory pathways with a convenient oral formulation."
Dr. Vipul Jairath, a Professor of Medicine at Western University in Canada and Chair of Sorriso Pharmaceuticals’ Scientific Advisory Board, expressed, "As both a clinician and researcher, I am excited about the advancements SOR102 is making in tackling the intricate pathophysiology of ulcerative colitis. The strategy of dual inhibition of TNFα and IL-23 through an oral medication is bold and innovative, representing significant promise for enhancing patient care. These Phase 1b results lay a strong groundwork for continued development and highlight the potential of Sorriso’s platform to create groundbreaking oral therapies."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
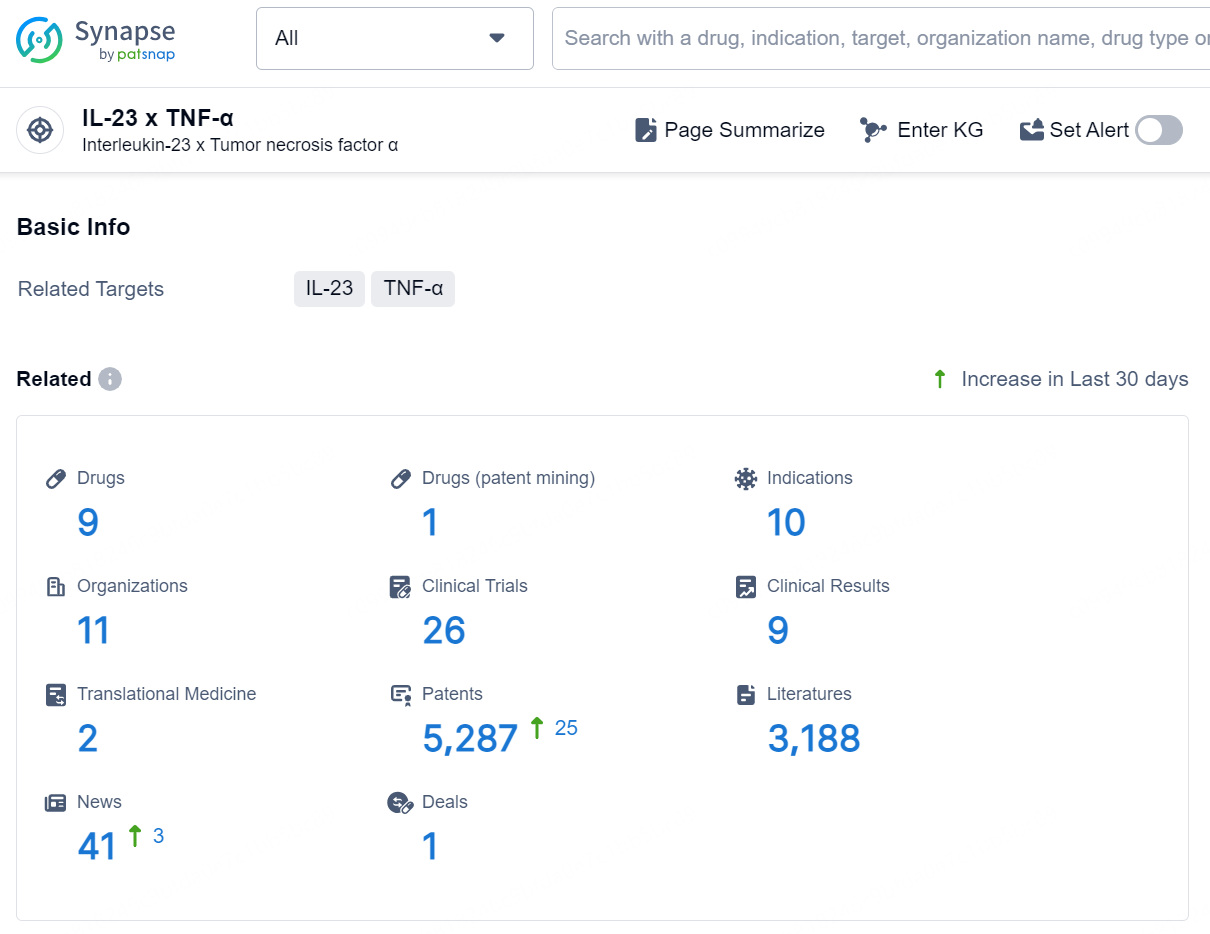
According to the data provided by the Synapse Database, As of December 24, 2024, there are 9 investigational drugs for the IL-23 and TNF-α target, including 10 indications, 11 R&D institutions involved, with related clinical trials reaching 26 and as many as 5287 patents.
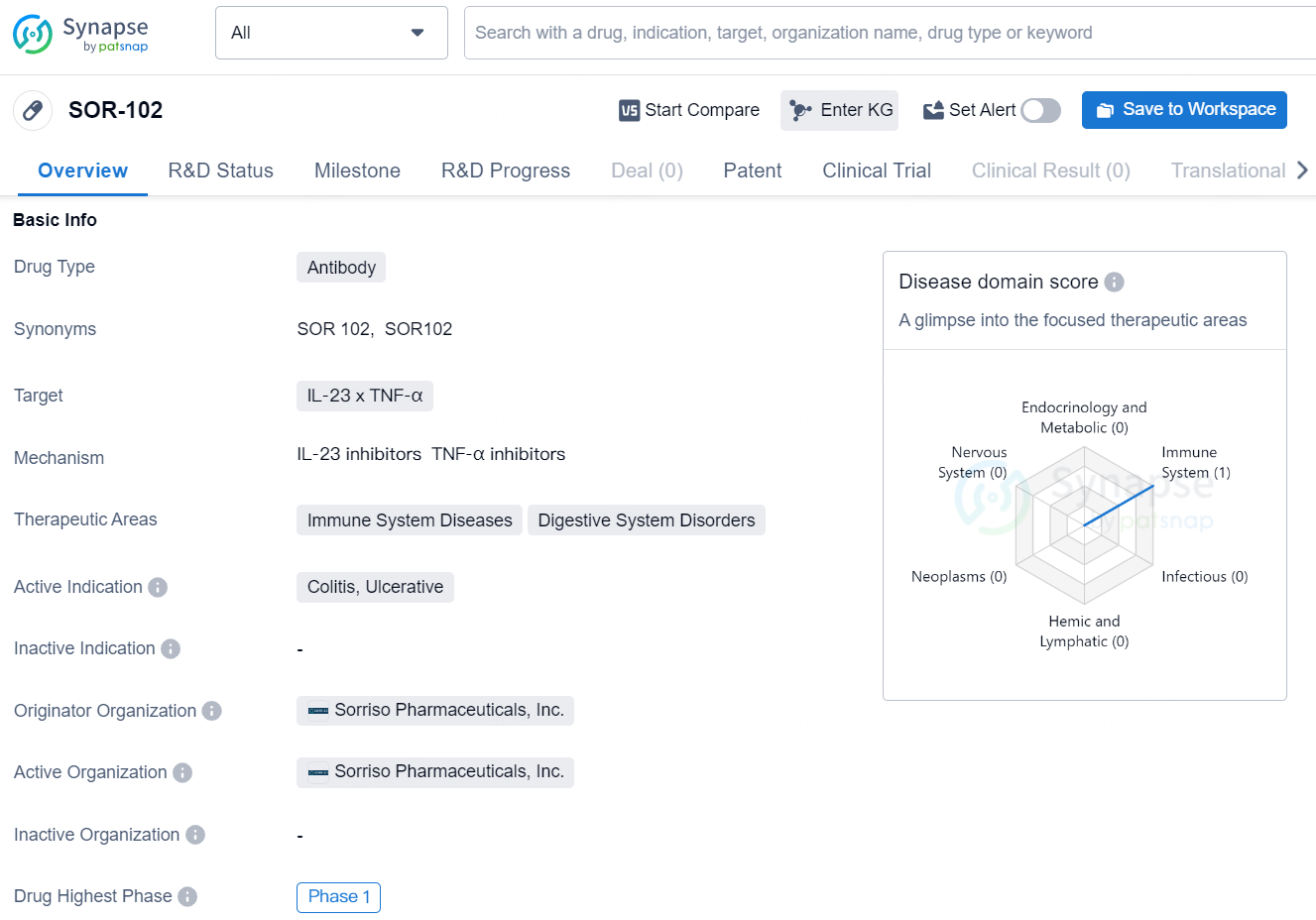
SOR-102 is an antibody drug designed to target IL-23 and TNF-α, with a focus on treating immune system diseases and digestive system disorders. The drug is currently in Phase 1 of clinical trials and is being developed by Sorriso Pharmaceuticals, Inc. The specific therapeutic areas for SOR-102 include colitis and ulcerative conditions.