FDA-Approved Combination of Meloxicam and Rizatriptan Offers Dual-Action Relief for Migraine Treatment
The combination drug Meloxicam and Rizatriptan, received approval from the U.S. Food and Drug Administration (FDA) on January 30, 2025, for the treatment of migraine.
Mechanism of Action
COX-2 inhibitors are a class of drugs specifically designed to reduce inflammation and pain by selectively inhibiting cyclooxygenase-2 (COX-2) enzyme. This enzyme is responsible for catalyzing the production of prostaglandins, which are chemical mediators involved in inflammation, pain, and fever. Compared to non-selective nonsteroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors exert minimal effects on COX-1, which helps reduce the risk of gastrointestinal side effects while maintaining effective anti-inflammatory and analgesic properties. These drugs are commonly used to treat mild to moderate pain in conditions such as osteoarthritis and rheumatoid arthritis and are particularly beneficial for patients who cannot tolerate traditional NSAIDs or are at high risk of developing gastrointestinal complications.
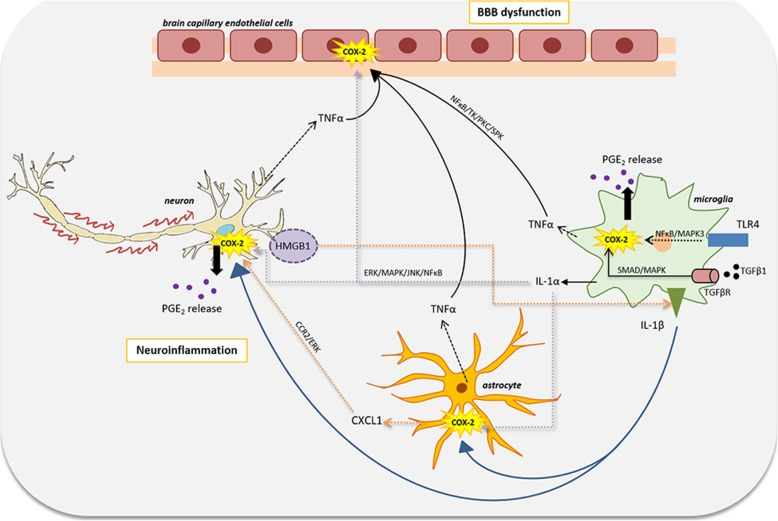
Beyond pain relief and inflammation control, COX-2 inhibitors have also shown potential in tumor prevention and treatment. Studies indicate that COX-2 is overexpressed in various human cancers and plays a role in tumor initiation and progression. As a result, COX-2 inhibitors might contribute to cancer prevention or serve as adjunctive therapies. For example, Celecoxib has been studied for its potential benefits in patients with familial adenomatous polyposis (FAP), demonstrating certain anticancer effects.
Meloxicam as a Selective COX-2 Inhibitor
Meloxicam is a selective COX-2 inhibitor that reduces prostaglandin synthesis by selectively inhibiting COX-2 enzyme activity. This leads to reduced pain, inflammation, and fever. Due to its higher selectivity for COX-2 over COX-1, Meloxicam may have a lower risk of gastrointestinal side effects compared to traditional non-selective NSAIDs.
While Meloxicam helps mitigate NSAID-associated gastrointestinal risks, caution is still necessary during its use, as it is not entirely risk-free. Some patients may experience mild gastrointestinal discomfort, such as dyspepsia, nausea, or abdominal pain. In rare but severe cases, complications such as ulcers, gastrointestinal bleeding, or perforation may occur.
The 5-hydroxytryptamine 1D (5-HT1D) receptor and 5-hydroxytryptamine 1B (5-HT1B) receptor are G-protein-coupled receptors (GPCRs) that belong to the serotonin (5-HT) family. These receptors play critical roles in the central nervous system (CNS), particularly in the regulation of pain, mood, behavior, and other physiological functions. In migraine treatment, 5-HT1B and 5-HT1D receptor agonists have been proven highly effective.
Rizatriptan as a 5-HT1B/1D Receptor Agonist
Rizatriptan is a 5-HT1B/1D receptor agonist and a key medication for the acute treatment of migraine. It exerts its effects by binding to 5-HT1B and 5-HT1D receptors, which are present in intracranial blood vessels, vascular smooth muscle cells, and trigeminal nerve endings. Upon receptor activation, Rizatriptan induces cranial vasoconstriction and inhibits the release of calcitonin gene-related peptide (CGRP), a key inflammatory mediator involved in migraine pathophysiology.
Beyond its vasoconstrictive properties, Rizatriptan also modulates pain signal transmission by acting on specific receptors within the pain-processing pathways of the CNS. This suggests that Rizatriptan may have direct central effects in migraine relief. Its multifaceted mechanism of action helps alleviate migraine symptoms, including headache, nausea, vomiting, and hypersensitivity to light and sound. Clinical studies have demonstrated that Rizatriptan provides rapid symptom relief, particularly when administered early during a migraine attack, significantly improving patients' quality of life.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
- 1. Rawat C, Kukal S, Dahiya UR, Kukreti R. Cyclooxygenase-2 (COX-2) inhibitors: future therapeutic strategies for epilepsy management. J Neuroinflammation. 2019 Oct 30;16(1):197. doi: 10.1186/s12974-019-1592-3. PMID: 31666079; PMCID: PMC6822425.