Finotonlimab: A Promising Anti-PD-1 Antibody for Solid Tumors
Finotonlimab, developed by Sinocelltech, is a recombinant humanized anti-programmed death receptor-1 (PD-1) monoclonal antibody. On February 8, 2025, it received NMPA approval for use in combination with platinum-based chemotherapy as a first-line treatment for recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN). Additionally, it is being investigated for the treatment of various solid tumors, including lymphoma, esophageal squamous cell carcinoma, colorectal cancer, gastric adenocarcinoma, and squamous non-small cell lung cancer (NSCLC).
Programmed death receptor-1 (PD-1) is a protein expressed on the surface of activated T cells. Its ligands, PD-L1 and PD-L2, are expressed on various cell types, including tumor cells and non-malignant cells in the tumor microenvironment. Under normal physiological conditions, when a T cell recognizes a specific antigen through the T cell receptor (TCR), it receives an activation signal. However, if PD-1 on the T cell interacts with PD-L1 or PD-L2 on adjacent cells (such as tumor cells), it transmits an inhibitory signal. This leads to:
·Suppression of T cell function,
·Reduced IL-2 production and T cell proliferation,
·Weakened effector function and survival of T cells.
This mechanism is essential for maintaining immune tolerance and preventing autoimmune diseases.
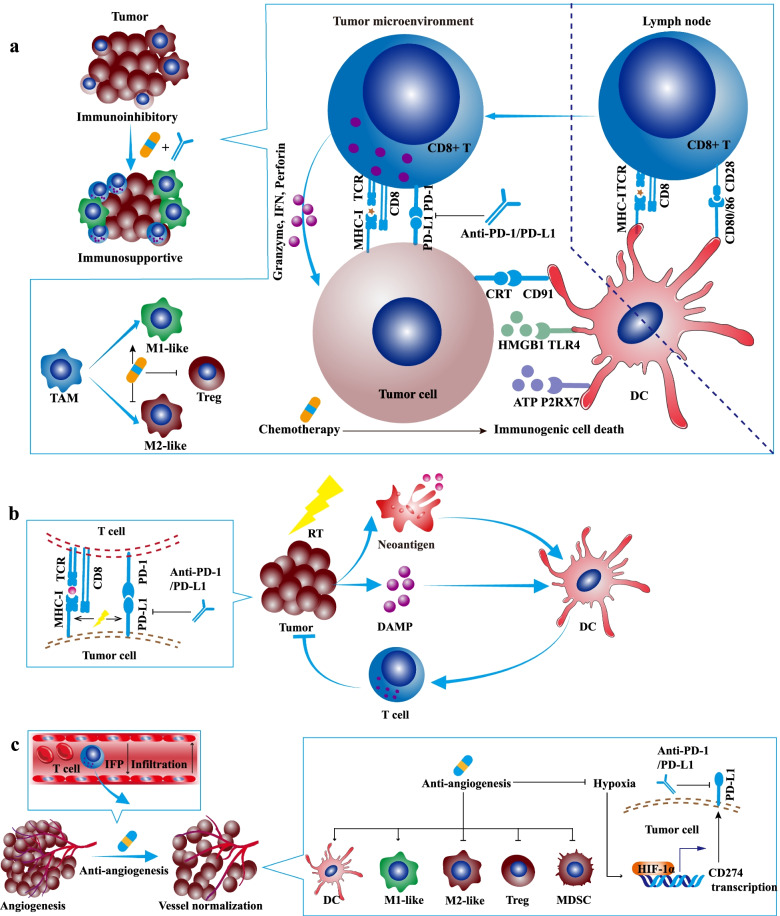
However, in cancer, tumor cells often upregulate PD-L1 expression, exploiting this pathway to inhibit T cell activity and evade immune surveillance. As a result, even tumor-specific T cells may become functionally exhausted due to PD-1/PD-L1 signaling, reducing their ability to eliminate cancer cells.
Finotonlimab works by specifically binding to PD-1 on T cells, preventing its interaction with PD-L1 and PD-L2. This reverses T cell suppression, allowing them to recognize and destroy cancer cells effectively. Additionally, studies suggest that blocking the PD-1/PD-L1 pathway not only directly activates T cells but also modifies the tumor microenvironment, reducing the proportion of regulatory T cells (Tregs) and further enhancing anti-tumor immune responses.
Clinical Data and Therapeutic Potential
Finotonlimab has demonstrated significant potential in clinical development. A Phase III clinical trial showed that when Finotonlimab was combined with cisplatin and 5-fluorouracil (C5F) chemotherapy, it significantly improved overall survival (OS) from 10.5 months to 14.1 months compared to chemotherapy alone. Moreover, the objective response rate (ORR) increased from 29.4% to 39.9%, highlighting its potential as a more effective treatment option for certain cancer patients.
Sinocelltech has also submitted a new indication application for the combination of Finotonlimab with a bevacizumab biosimilar as a first-line treatment for hepatocellular carcinoma (HCC), which has been accepted for review.
Safety and Considerations
As with other immune checkpoint inhibitors, Finotonlimab may cause immune-related adverse events (irAEs), including rash, pruritus, and thyroid dysfunction. Close monitoring and appropriate management strategies are essential to mitigate these risks. Physicians must consider individual patient differences and adjust treatment plans accordingly to optimize safety and efficacy.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022 Jan 21;21(1):28. doi: 10.1186/s12943-021-01489-2. PMID: 35062949; PMCID: PMC8780712.