Prusogliptin: A Novel DPP-4 Inhibitor Approved in China
Prusogliptin, developed by CSPC Ouyi Pharmaceutical, is a novel oral dipeptidyl peptidase-4 (DPP-4) inhibitor that was approved by National Medical Products Administration (NMPA) on January 8, 2025. This approval marks its official entry into the Chinese market, providing a new treatment option for patients with type 2 diabetes.
Mechanism of Action
DPP-4 (dipeptidyl peptidase-4) plays a key role in regulating the metabolism of two important incretin hormones—glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These hormones are crucial in blood glucose regulation, particularly in postprandial glucose control.
After food intake, the intestine releases GLP-1 and GIP, which stimulate insulin secretion from pancreatic β-cells and inhibit glucagon secretion from pancreatic α-cells (glucagon is a hormone that increases blood sugar levels). This mechanism helps to reduce postprandial blood glucose spikes. However, GLP-1 and GIP have very short half-lives, as they are rapidly degraded and inactivated by the DPP-4 enzyme.
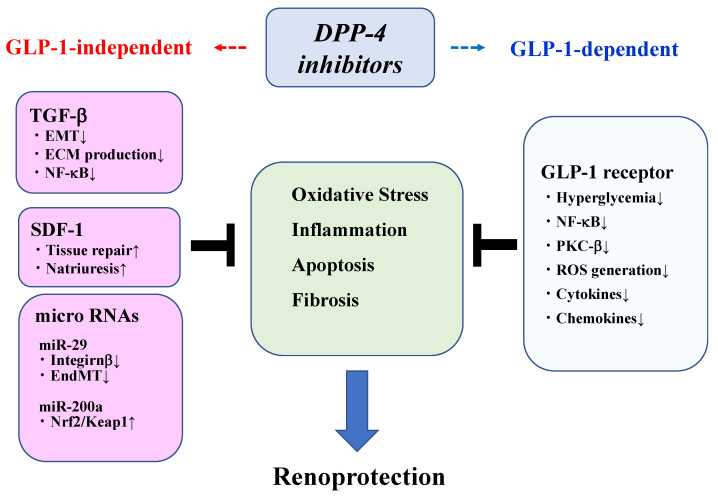
Prusogliptin inhibits the activity of the DPP-4 enzyme, preventing the rapid degradation of GLP-1 and GIP. As a result, these incretin hormones remain active for a longer period, allowing them to more effectively:
·Stimulate insulin secretion,
·Suppress glucagon secretion, and
·Ultimately, lower blood glucose levels.
Because this mechanism is glucose-dependent (only activated when blood glucose levels are elevated), DPP-4 inhibitors do not carry the risk of hypoglycemia, unlike traditional insulin secretagogues.
Additionally, Prusogliptin enhances pancreatic β-cell glucose sensitivity, reduces unnecessary glucagon secretion, and helps maintain blood glucose within a healthy range. Unlike traditional insulin secretagogues, Prusogliptin does not directly stimulate insulin release but rather enhances endogenous GLP-1 activity, resulting in a lower risk of hypoglycemia and less likelihood of weight gain. This makes it an important advantage for the long-term management of type 2 diabetes.
Prusogliptin has demonstrated a safety profile comparable to existing DPP-4 inhibitors, with a low incidence of adverse reactions and minimal drug interactions. Furthermore, for patients with mild to moderate renal impairment, dose adjustment is not required, which enhances its clinical applicability and convenience.
These characteristics make Prusogliptin an ideal treatment option for type 2 diabetes patients—particularly those seeking to avoid hypoglycemia risk and weight gain. With its launch in the Chinese market, more type 2 diabetes patients now have access to this novel therapeutic approach, improving their long-term diabetes management.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
- 1. Li D, Shi W, Wang T, Tang H. SGLT2 inhibitor plus DPP-4 inhibitor as combination therapy for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2018 Aug;20(8):1972-1976. doi: 10.1111/dom.13294. Epub 2018 Apr 14. PMID: 29573110.