FDA Approves First T-Cell Engager Therapy for Extensive-Stage Small Cell Lung Cancer
Amgen revealed that IMDELLTRA™ (tarlatamab-dlle) has received approval from the U.S. Food and Drug Administration for use in adult patients experiencing disease progression in extensive-stage small cell lung cancer following platinum-based chemotherapy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
IMDELLTRA has been granted accelerated approval due to the promising response rate and duration observed in clinical trials. Ongoing approval for this use might depend on the confirmation and detailed analysis of clinical benefits in a confirmatory study.
"The FDA's approval of IMDELLTRA is a significant breakthrough for patients struggling with ES-SCLC. This DLL3-targeting therapy for ES-SCLC offers a groundbreaking option that shows enduring responses in pretreated patients," stated Jay Bradner, M.D., executive vice president of Research and Development and chief scientific officer at Amgen.
"This approval underscores our dedication to tackling aggressive cancers through our second FDA-approved Bispecific T-cell Engager molecule. IMDELLTRA provides hope to these patients in dire need of novel therapies, and we take pride in delivering this much-anticipated effective treatment to them," Jay Bradner continued.
"Lung cancer is a complex and devastating condition, with less than 3% of individuals with ES-SCLC surviving beyond five years," said David P. Carbone, M.D., Ph.D., professor of internal medicine and director of the James Thoracic Oncology Center at Ohio State University Medical Center. "In the DeLLphi-301 trial, the median overall survival was 14.3 months, with 40% of patients responding to treatment with tarlatamab. These durable responses signify a major advancement in the SCLC treatment paradigm."
IMDELLTRA is the first DLL3-targeting Bispecific T-cell Engager therapy that activates the patient’s T cells to attack DLL3-expressing tumor cells.
"Following decades of minimal progress in the SCLC treatment landscape, an effective and innovative treatment option is now accessible," noted Laurie Fenton Ambrose, co-founder, president, and CEO of GO2 for Lung Cancer. “Today’s FDA approval is a critical milestone for the SCLC community, as the availability of a targeted bispecific therapy introduces new possibilities for those suffering from this aggressive disease.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
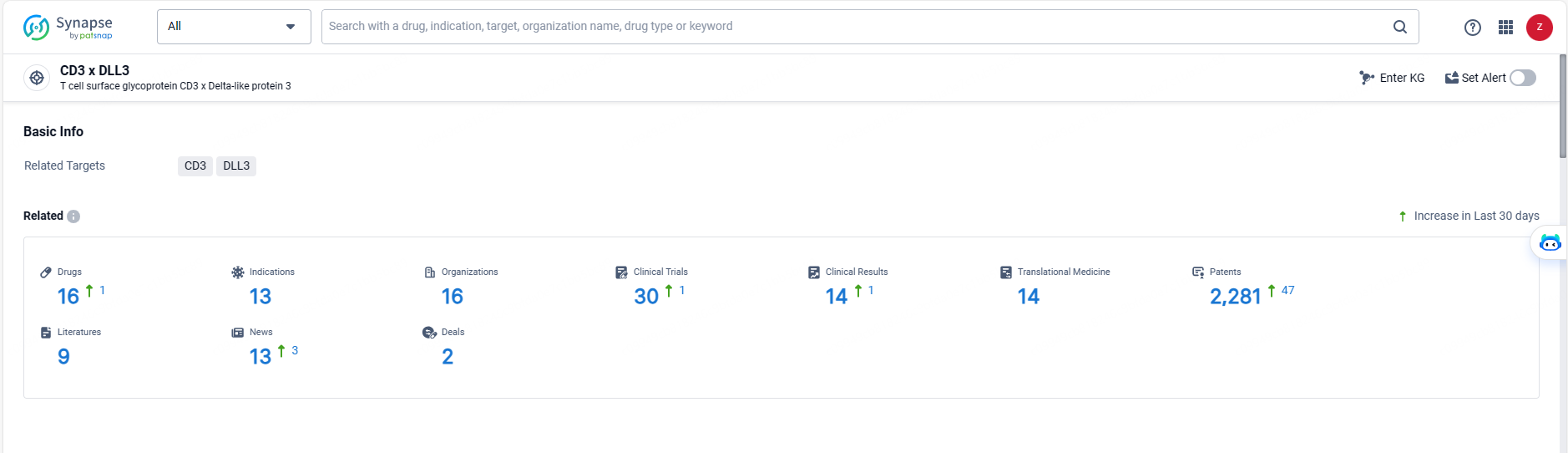
According to the data provided by the Synapse Database, As of May 20, 2024, there are 16 investigational drugs for the CD3 and DLL3 target, including 13 indications, 16 R&D institutions involved, with related clinical trials reaching 30, and as many as 2281 patents.
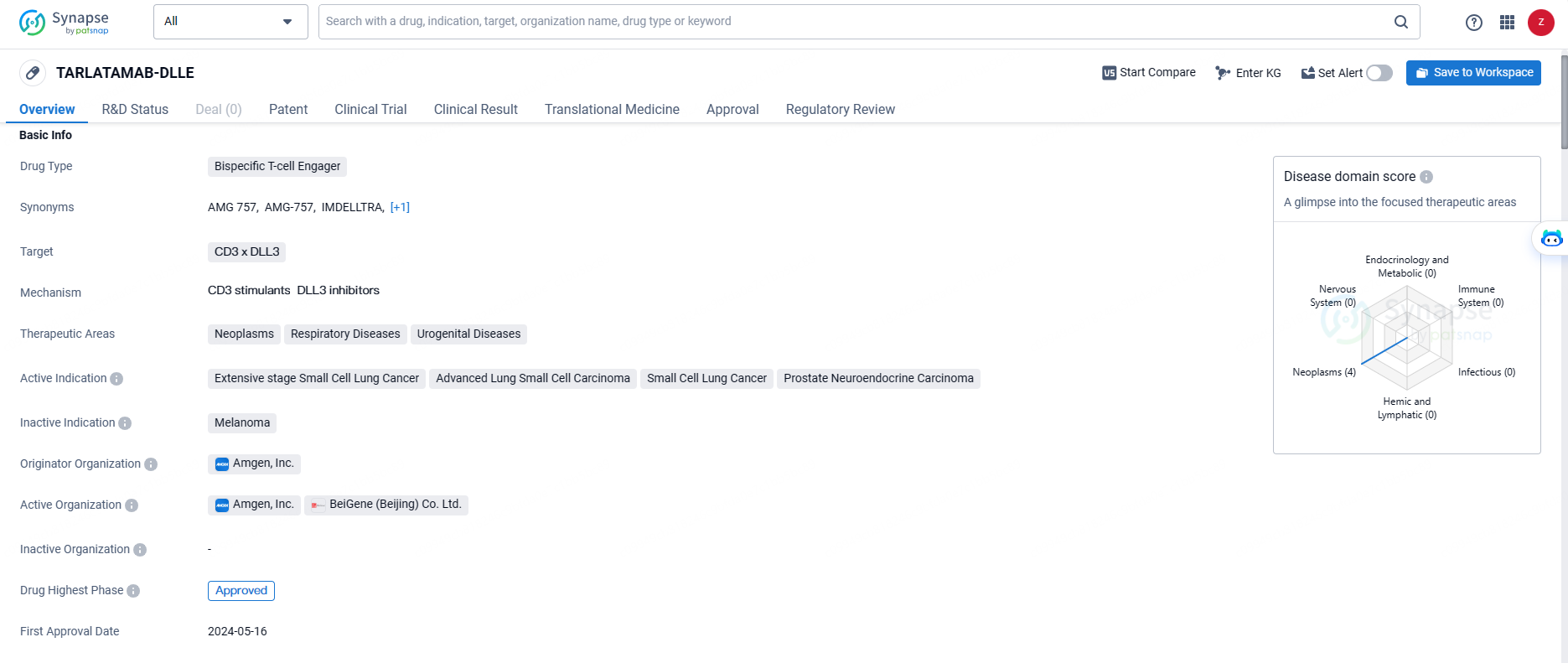
TARLATAMAB-DLLE targets CD3 x DLL3 and is indicated for the treatment of neoplasms, respiratory diseases, and urogenital diseases. Its active indications include extensive stage small cell lung cancer, advanced lung small cell carcinoma, small cell lung cancer, and prostate neuroendocrine carcinoma.