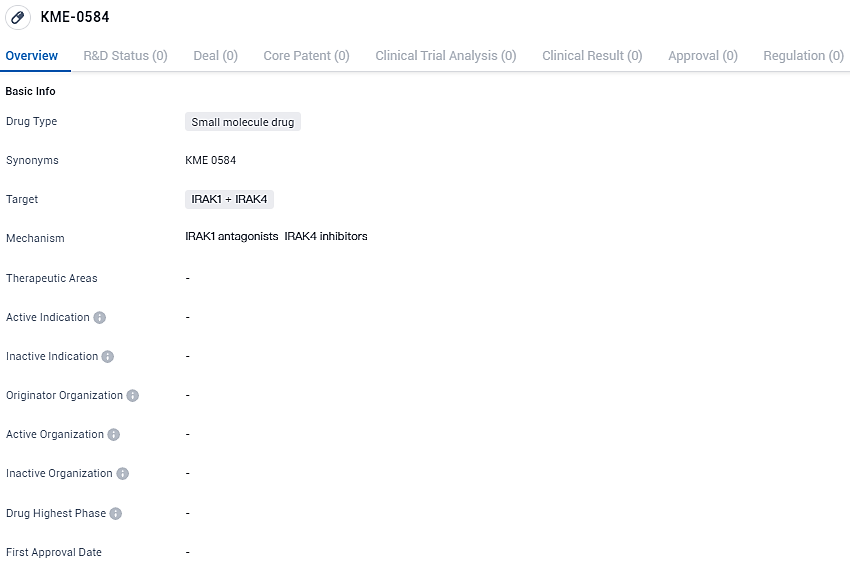
FDA Approves Kurome's Clinical Trials for AML and Advanced MDS Drug KME-0584
Kurome Therapeutics Inc. is excited to report that the IND for KME-0584 has been approved by the U.S. Food and Drug Administration. This approval grants the organization the authorization to move forward with a Phase 1 clinical study targeting patients with relapsed or refractory AML and those diagnosed with high-risk MDS. The commencement of the clinical trial by Kurome is scheduled for the second half of 2024.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Reaching this significant point is a noteworthy achievement for the team at Kurome and our partners, affirming our distinct strategy for addressing AML and MDS, and confirming the readiness of KME-0584 for early phase clinical trials," announced Jan Rosenbaum, Ph.D., the chief executive and science officer of Kurome Therapeutics.
Jan Rosenbaum expressed eagerness to commence the clinical trial, focusing on the novel treatment strategy which aims to disrupt abnormal immune signaling in both AML and high-risk MDS by concurrently inhibiting IRAK1 and IRAK4. This approach aims to enhance treatment outcomes for patients with relapsed/refractory diseases who are often hard to treat.
KME-0584 stands out because of its strong activity and specificity as an inhibitor, targeting IRAK1 and IRAK4, and all variations of the FLT3 gene, which contributes to the progression of relapsed/refractory AML or high-risk MDS. Designed for oral use, KME-0584 can be used both as a stand-alone therapy and in combination with other drugs like azacitidine or venetoclax.
The initial phase of the clinical study will investigate the safety profile, tolerability, pharmacological behavior, and therapeutic potential of KME-0584, both as a unique treatment and in drug combinations, for individuals with AML and high-risk MDS. This phase of the trial will progressively increase dosages and will broaden to encompass as many as 100 participants. Kurome Therapeutics is targeting the commencement of this trial for the year 2024, and it is set to take place at several research locations throughout the United States.
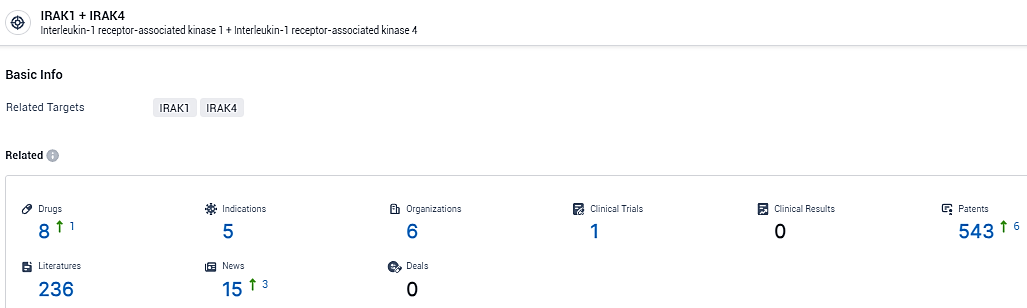
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 22, 2024, there are 8 investigational drugs for the IRAK1 and IRAK4 target, including 5 indications, 6 R&D institutions involved, with related clinical trials reaching 1, and as many as 543 patents.
By targeting IRAK1 and IRAK4, KME-0584 may have the potential to regulate immune responses and inflammation, which could be beneficial in various disease conditions. Further research and development are required to fully understand the drug's efficacy and safety profile.