Teva Unveils Fresh Evidence for Anti-TL1A ('574) Antibody's Safe Use, Comfort and Efficacy at ECCO's 2024 Event
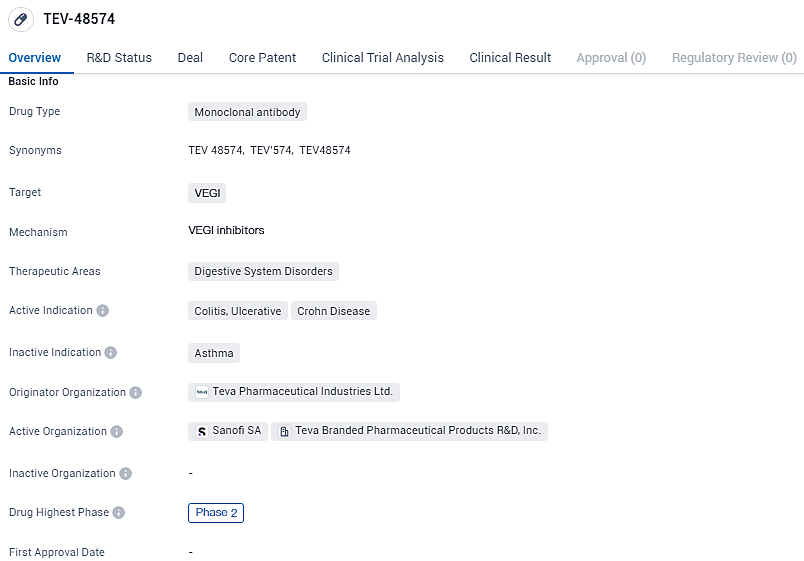
Teva Pharmaceuticals has released encouraging initial results concerning the safety, patient tolerance, and pharmacokinetic profile of its investigational product known as anti-TL1A (TEV-’574). This human IgG1 monoclonal antibody, which is directed at the TNF-like ligand 1A, shows promise to stand out in its class owing to its dual-action capability to suppress inflammation and inhibit fibrotic tissue formation.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Research outcomes demonstrate that the quick and enduring reduction of unbound TL1A validates the interaction with the anti-TL1A target. The evidence reveals an acceptable safety threshold for asthmatic individuals, bolstering the rationale for further clinical trials in moderate-to-severe inflammatory bowel disease (IBD), specifically ulcerative colitis and Crohn's disease. These insights will be shared at the forthcoming 19th Annual Congress of the European Crohn’s and Colitis Organisation, scheduled for February 21-24, 2024, in Stockholm, Sweden.
"The initial human studies on anti-TL1A have yielded promising results, clearly indicating effective engagement with the TL1A target, signifying a positive safety profile, and acceptance in the body. These findings are in line with our extensive pre-clinical research and strengthen the case for ongoing clinical investigations of anti-TL1A for IBD treatment. In IBD, TL1A significantly heightens the immune response, causing severe inflammation and fibrotic damage within the digestive system," explained Dr. Eric Hughes, Teva's Executive Vice President of Global R&D and Chief Medical Officer.
Dr. Hughes elaborated, "Presently, we are examining anti-TL1A's effectiveness and safety in the context of IBD through our Phase 2 RELIEVE UCCD trial, which utilises a basket study model for efficiency and inclusivity, allowing the participation of patients with either form of IBD. The emerging data suggest anti-TL1A (TEV-574) could potentially serve as a groundbreaking therapeutic approach for IBD, and we are dedicated to advancing innovative treatments to enhance the quality of life for those afflicted with IBD with expedited availability."
On November 30, 2023, Teva and Sanofi publicized the finalization of their alliance to jointly progress and market anti-TL1A for treating UC and CD, which are types of IBD. Under the collaboration agreement terms, Teva was granted an initial payment of $500 million after the partnership's conclusion and can anticipate up to $1 billion dependent on meeting specified developmental and commercial milestones.
Both companies will evenly distribute global development expenses, as well as profits and losses in key markets; Teva is responsible for introducing the product in Europe, Israel, and other selected regions, while Sanofi will oversee launches in North America, Japan, additional Asian territories, and other global markets, with Teva receiving royalties in these locations. Sanofi is tasked with spearheading the Phase 3 program's development.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
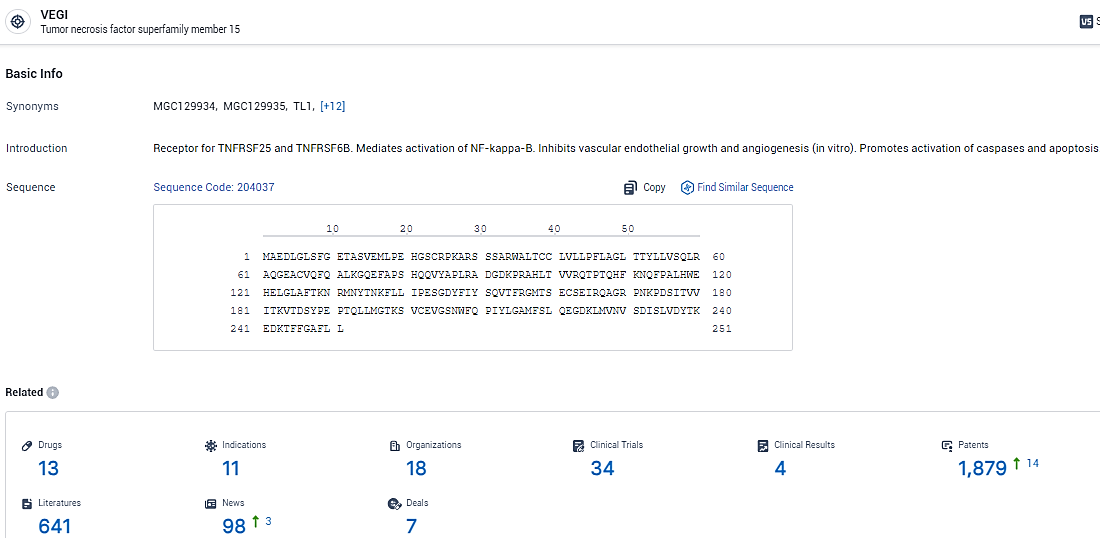
According to the data provided by the Synapse Database, As of February 26, 2024, there are 13 investigational drugs for the TL1 target, including 11 indications, 18 R&D institutions involved, with related clinical trials reaching 34, and as many as 1879 patents.
TEV-’574 is a potentially best-in-class human IgG1 monoclonal antibody that targets tumor necrosis factor (TNF)-like ligand 1A (TL1A), also known as TNF superfamily member 15. TL1A signaling is believed to amplify inflammation and drives fibrosis associated with asthma and inflammatory bowel disease; thus, targeting TL1A with TEV-’574 may mitigate the over-active immune response in these conditions. The drug is currently in Phase 2 of clinical development, indicating its potential as a future therapeutic option for patients suffering from these conditions.