FDA Approves Merck's WINREVAIR™ for Adult Pulmonary Arterial Hypertension
The pharmaceutical giant Merck, recognized as MSD in nations beyond the U.S. and Canadian borders, made a public statement concerning the recent greenlight from the U.S. FDA for their drug sotatercept-csrk, marketed under the trade name WINREVAIR™, which comes in injectable forms of 45mg and 60mg. This particular medication has been sanctioned for its use in adult patients who are struggling with pulmonary arterial hypertension. The drug aims to enhance the capacity for physical exertion, elevate the WHO functional class standing, and diminish the likelihood of facing adverse clinical progression events.
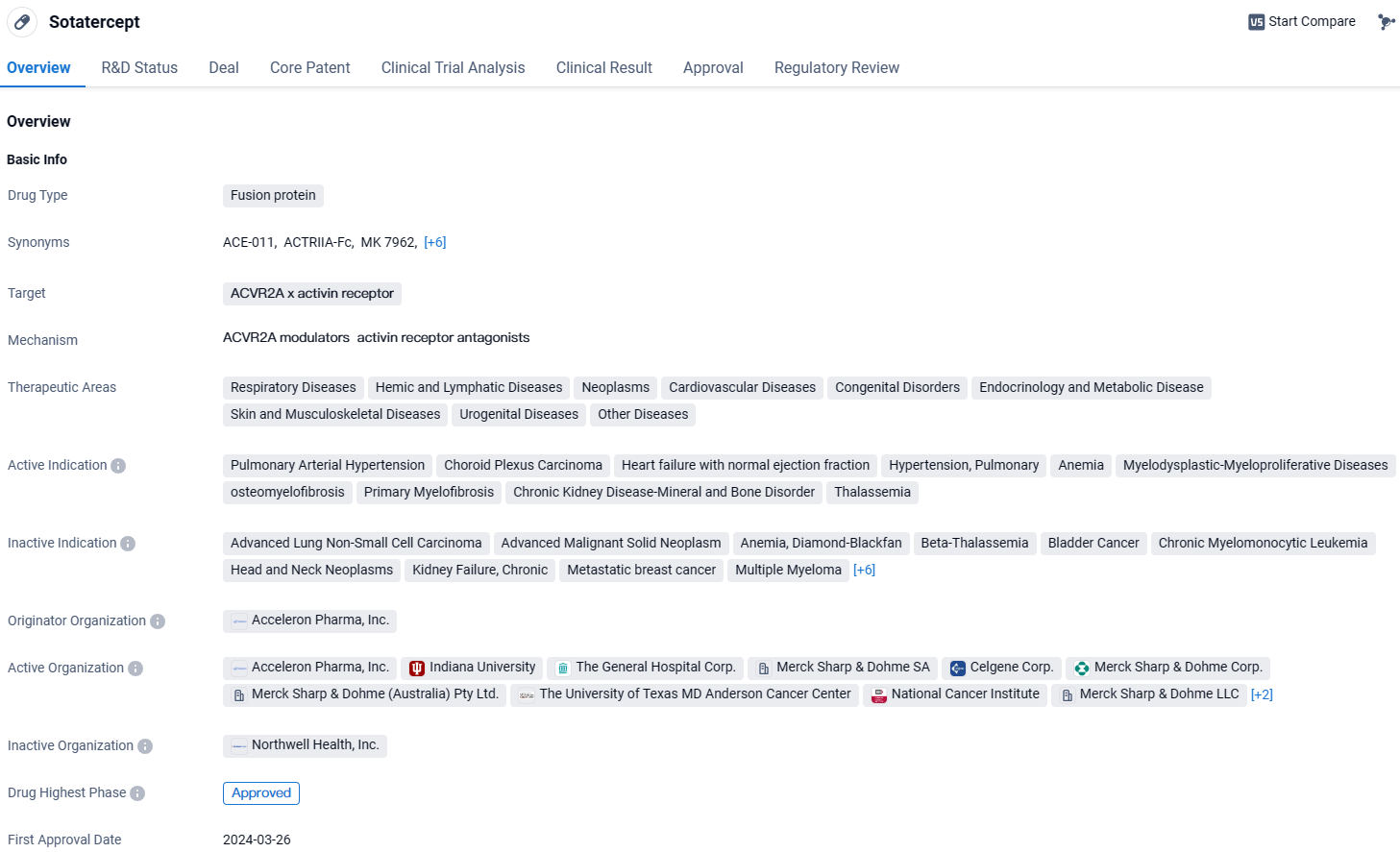
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
WINREVAIR received the FDA's Breakthrough Therapy Designation earlier. It introduces a novel intervention as the pioneering activin receptor ligand trap approved for the treatment of PAH—a condition that challenges existing therapies. WINREVAIR operates by altering the dynamics of growth signals within vascular cells, aiming to regulate the pathological cellular overgrowth associated with PAH.
Medical practitioners are advised to assess patients' hemoglobin and platelet levels before administering each of the initial five doses of WINREVAIR, extending the monitoring if abnormal results persist, and intermittently afterwards to see if adjustments in the dosing are necessary. An observed potential effect of WINREVAIR is the elevation of hemoglobin, potentially triggering erythrocytosis. In severe cases, this condition may elevate the likelihood of thromboembolic events or a state of hyperviscosity.
Additionally, a reduction in platelets brought on by WINREVAIR could invoke severe thrombocytopenia, heightening the chances of bleeding. This risk was noted to be especially heightened in patients who concurrently underwent prostacyclin therapy.
“Pulmonary arterial hypertension patients require continuing evolutions in treatment to attain crucial health outcomes, such as enhanced physical activity and improved disease status," remarked Dr. Aaron Waxman of Brigham and Women’s Hospital, who acted as a research lead in the crucial Phase 3 STELLAR study. "With the addition of sotatercept to existing medication regimens, we’re looking at a potential new paradigm in PAH patient care.”
“Despite advances, PAH continues to be a significantly harmful illness, leading to elevated morbidity and mortality rates,” commented Dr. Eliav Barr, a leading figure at Merck Research Laboratories. “The authorization of WINREVAIR marks a pivotal point, reflecting our commitment to rigorous scientific pursuit and our dedication to addressing the rare condition of PAH. We take pride in being able to offer this innovative treatment to those in need.”
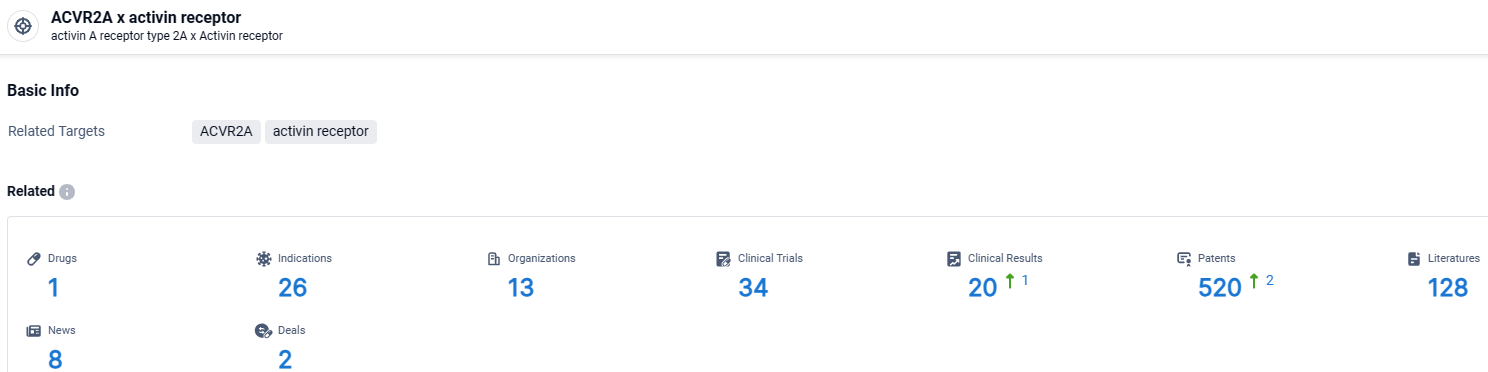
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 28 2024, there are 1 investigational drugs for the ACVR2A and activin receptor target, including 26 indications, 13 R&D institutions involved, with related clinical trials reaching 34, and as many as 520 patents.
Sotatercept targets the ACVR2A and activin receptor and has shown promising therapeutic potential in various therapeutic areas. It has been approved globally and received its first approval in the United States. The drug is currently in Phase 1 of development in China. With its regulatory designations, Sotatercept is expected to address critical medical needs and provide significant benefits to patients suffering from the approved indications.