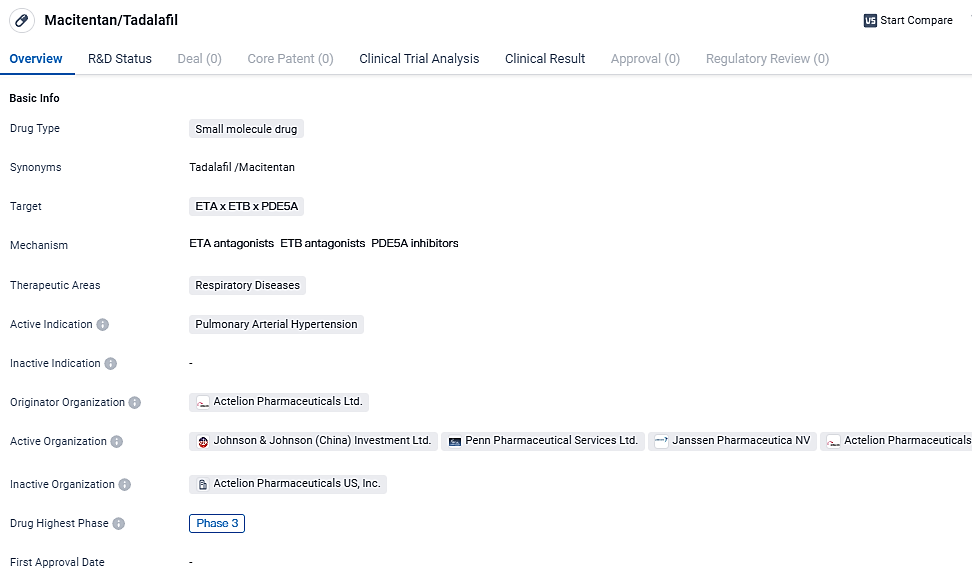
FDA Approves OPSYNVI® as First Daily Combo Therapy for PAH
Johnson & Johnson has disclosed the authorization by the U.S. Food and Drug Administration of a new medication, OPSYNVI®, which is a singular tablet formulation composed of two active agents: macitentan, which serves as an antagonist to endothelin receptors, and tadalafil, an inhibitor targeting phosphodiesterase 5 (PDE5). This pharmaceutical product is sanctioned for the ongoing management of pulmonary arterial hypertension in adult patients whose condition is classified within the WHO functional class II to III range.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
OPSYNVI® can be prescribed for individuals with Pulmonary Arterial Hypertension (PAH) who have not received treatment previously or for those currently on treatment with an Endothelin Receptor Antagonist (ERA), a Phosphodiesterase type 5 (PDE5) inhibitor, or a combination of both classes of medication. It is appropriate for those receiving coordinated treatment with stable regimens of macitentan at 10 mg and tadalafil at 40 mg delivered as individual tablets.
PAH is an uncommon, progressively worsening condition posing severe risks, which occurs due to tightening of the smaller vessels in the lungs causing increased pressure within the pulmonary circulation, potentially leading to failure of the right side of the heart. The annual incidence of PAH in the United States is between 500 and 1,000, which confirms its status as a rare disease.
The 2022 guidelines from the European Society of Cardiology/European Respiratory Society endorse the use of a combined initial therapy involving an ERA and a PDE5 inhibitor for individuals with idiopathic PAH, heritable or drug-associated PAH, or PAH related to connective tissue disease who do not have significant cardiopulmonary diseases and are considered to be at low to intermediate risk.
The endorsement from the FDA for OPSYNVI® comes after the significant A DUE Phase 3 study outcomes, where OPSYNVI® showed a superior decrease in Pulmonary Vascular Resistance in comparison to monotherapies of tadalafil or macitentan over a period of 16 weeks. OPSYNVI® includes a prominent warning related to potential harm to embryos and fetuses, obligating female patients to register in the Macitentan-Containing Products Risk Evaluation and Mitigation Strategy program.
Dr. James F. List, M.D., Ph.D., the Global Therapeutic Area Head for Johnson & Johnson, with oversight of Pulmonary Hypertension and several other programs, expressed excitement over the introduction of OPSYNVI® in tablet form. This innovation is aimed at simplifying medication routines for individuals with PAH, a population often challenged by the complexity and volume of their daily medication intake, and it aligns with the latest guidelines which advocate for early or initial combination therapy.
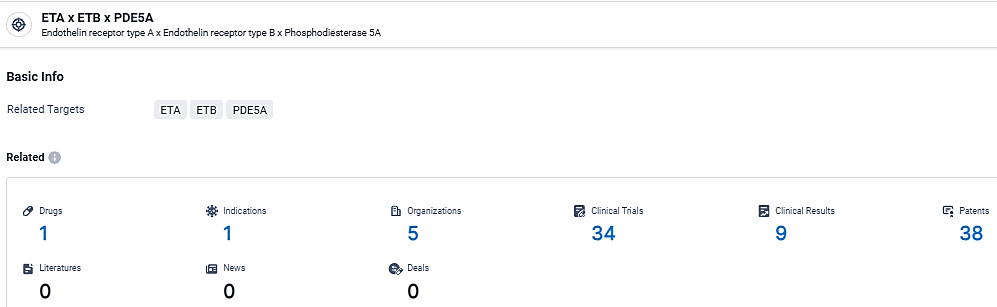
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 25, 2024, there are 1 investigational drugs for the ETB and ETA and PDE5A target, including 1 indications, 5 R&D institutions involved, with related clinical trials reaching 34, and as many as 38 patents.
OPSYNVI® is a small molecule drug that targets ETA, ETB, and PDE5A receptors. It is being developed for the treatment of pulmonary arterial hypertension. The drug aims to improve blood flow and reduce elevated blood pressure in the pulmonary arteries, addressing the symptoms associated with PAH. OPSYNVI® holds promise as a potential therapeutic option for patients suffering from this debilitating condition.