Ivonescimab Shows Promise Against Brain Metastases in NSCLC
Summit Therapeutics Inc. has unveiled encouraging results from its groundbreaking research on a unique bispecific antibody known as ivonescimab. This potential game-changer in its category is being showcased at the European Lung Cancer Congress in Prague, Czech Republic, in 2024. Attendees can view two posters that provide the latest insights into ivonescimab's performance from 12:00 to 12:45pm Central European Time during the conference. Additionally, these scientific posters will be accessible on the Summit Therapeutics Inc. official website following their initial display period at the congress.
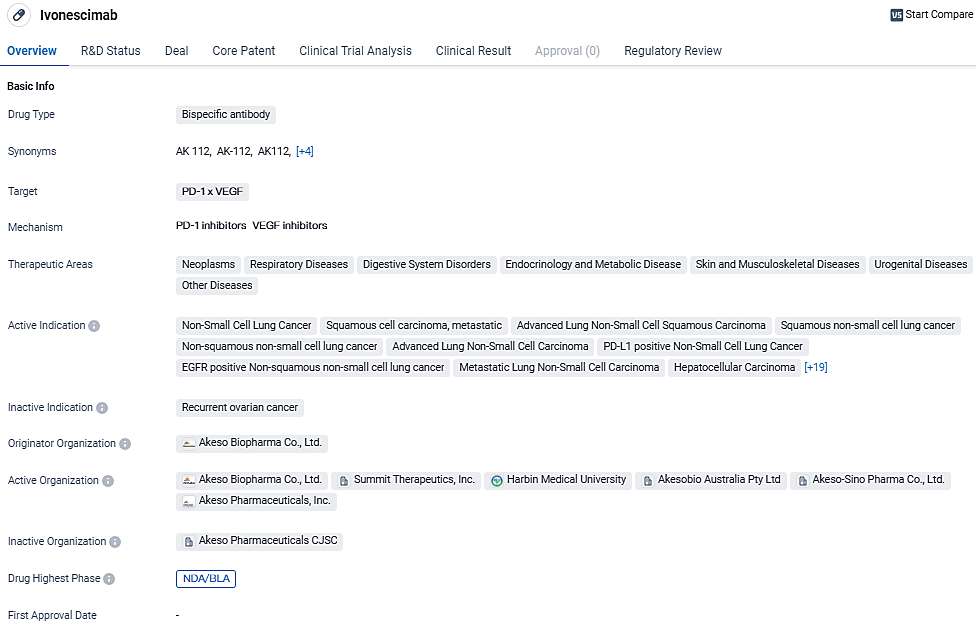
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The inaugural poster presentation, entitled "Evaluation of Ivonescimab as a Standalone Treatment or Paired with Platinum Dual-Agent Chemotherapy for Advanced Non-Small Cell Lung Cancer (NSCLC) Patients with Cerebral Metastatic Tumors," discusses insights from individuals who had undiagnosed cerebral metastases at the study's initiation. These subjects joined one of two separate Phase II clinical investigations: Trial AK112-202, where ivonescimab was administered solo, or Trial AK112-201, where ivonescimab was combined with a platinum-containing chemotherapy regimen, both targeting advanced or metastatic NSCLC patients.
Within this study, there were 35 participants identified with advanced or metastatic NSCLC and asymptomatic cerebral metastases at the beginning; 28 of these participants received ivonescimab in conjunction with chemotherapy as part of AK112-201, while the remaining seven were given ivonescimab as a singular therapy in AK112-202.
Dr. H. Jack West, the leading authority on clinical development at Summit, expressed satisfaction with the outcomes observed in the NSCLC subset with cerebral metastases treated with ivonescimab. He noted the commendable cerebral response rates, median duration of cerebral progression-free survival, and the encouraging signs of anti-cancer efficacy and tolerability. He also acknowledged the patient participants, the research teams, and the collaborative efforts with Akeso associates.
The sequel poster presentation, "Ivonescimab Phase 2 Outcomes: A Cutting-Edge PD-1/VEGF Dual-Functioning Agent in Union with Chemotherapy for Initial Management of Advanced/Metastatic NSCLC," showcases recent findings from AK112-201 Phase II study that focuses on a group treated with ivonescimab plus chemotherapy as a front-line approach for squamous and non-squamous advanced or metastatic NSCLC, explicitly excluding patients with specific genetic modifications amenable to targeted therapies.
The report also brings to light updated findings regarding NSCLC patients possessing EGFR mutations following tyrosine kinase inhibitor (TKI) therapy, and those who have been previously treated with a PD-(L)1 inhibitor alongside a chemotherapy doublet.
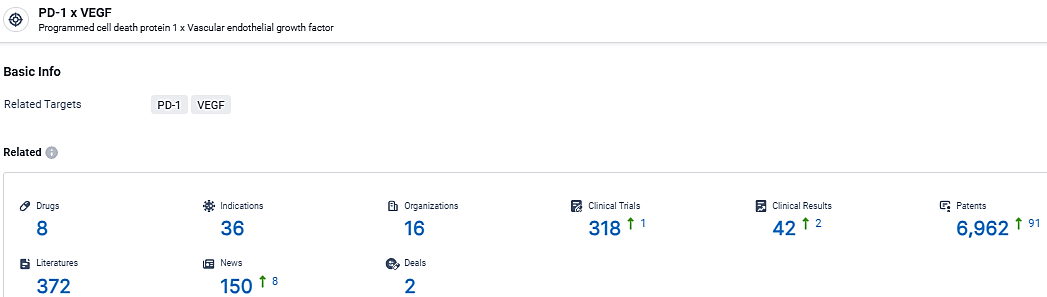
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 25, 2024, there are 8 investigational drugs for the PD-1 and VEGF target, including 36 indications, 16 R&D institutions involved, with related clinical trials reaching 318, and as many as 6962 patents.
Ivonescimab, known as SMT112 in Summit’s license territories, the United States, Canada, Europe, and Japan, and as AK112 in China and Australia, is an investigational, novel, potential first-in-class bispecific antibody combining the effects of immunotherapy via a blockade of PD-1 with the anti-angiogenesis effects associated with blocking VEGF into a single molecule. The drug is currently in the highest phase of development and has received priority review and breakthrough therapy designation.