FDA Approves Pfizer's Single-Dose Gene Therapy BEQVEZ™ for Adult Hemophilia B
Pfizer Inc. has revealed that the U.S. Food and Drug Administration granted approval for BEQVEZ™ (fidanacogene elaparvovec-dzkt), which is to be used for treating adult patients suffering from moderate to severe hemophilia B. This approval applies to those who are on factor IX (FIX) prophylactic treatment, or those who have experienced or currently experience life-threatening hemorrhages, or have histories of severe spontaneous bleeding. Additionally, it is required that patients do not show presence of neutralizing antibodies against the adeno-associated virus serotype Rh74var capsid, as confirmed by an FDA-approved assay.
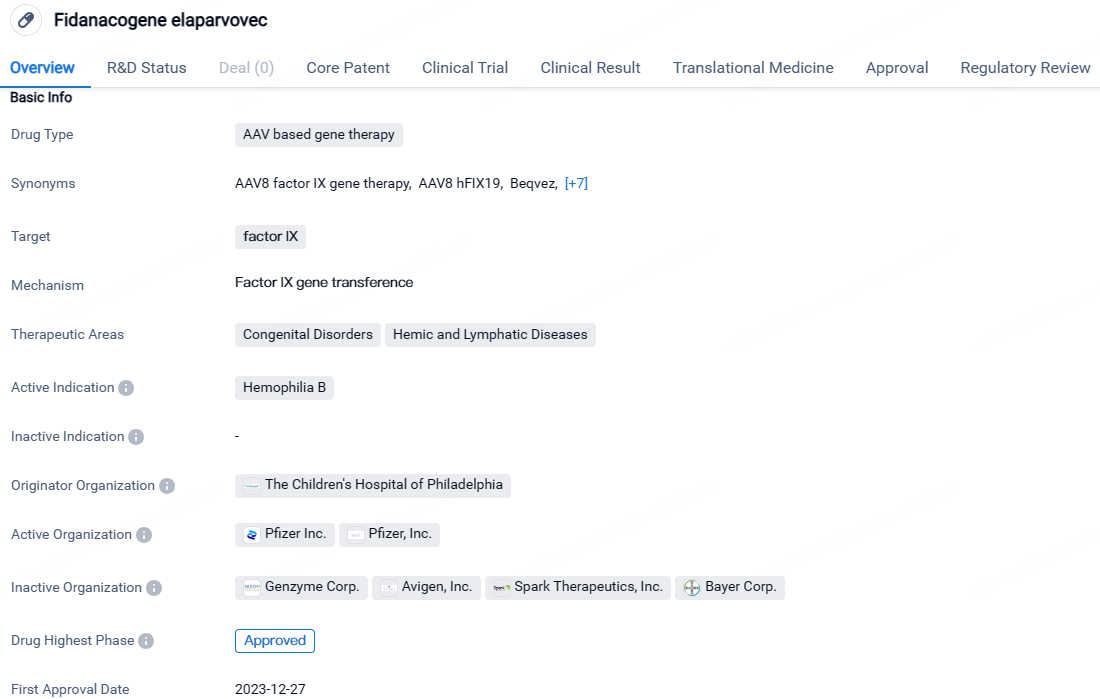
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
BEQVEZ represents a revolutionary single-dose therapy aimed at empowering individuals diagnosed with hemophilia B to autonomously produce FIX, moving away from the prevalent approach which mandates frequent intravenous FIX infusions, typically several times each week or month.
"Regular FIX infusions can be quite burdensome, disrupting daily activities for those affected by hemophilia B and frequently leading to unpredictable bleeding episodes. Such episodes can cause severe joint damage and impair mobility," remarked Adam Cuker, M.D., M.S., who is the Director of the Penn Comprehensive and Hemophilia Thrombosis Program. "A solitary application of BEQVEZ could significantly alter the management of the condition for suitable candidates by lessening the long-term health and treatment demands."
Currently, hemophilia B is managed through prophylactic FIX infusion therapies that act as a temporary replacement or boost for the deficient blood-clotting factor. However, even with routine prophylaxis, individuals with moderate to severe hemophilia B still face the threat of unexpected bleeding episodes.
The existing treatment protocols also exert considerable pressure on the financial and operational capacities of healthcare systems. The World Federation of Hemophilia notes that over 38,000 people globally live with hemophilia B.
Following the approval of BEQVEZ, Pfizer is initiating a pioneering warranty program that evaluates the longevity of patient responses. This program aims to offer greater assurance to healthcare financiers, enhance treatment accessibility for qualifying patients receiving BEQVEZ, and provide economic safeguards by covering potential treatment efficacy risks.
Managing hemophilia often significantly disrupts life, noted Kim Phelan, Chief Operating Officer at The Coalition for Hemophilia B. “An infusion of BEQVEZ might free up more time for patients to enjoy personal pursuits,” she stated. “We're thrilled to include BEQVEZ in our arsenal of potential treatments and are eager to see the outcomes it brings as we gather for our ongoing annual meeting in partnership with Pfizer.”
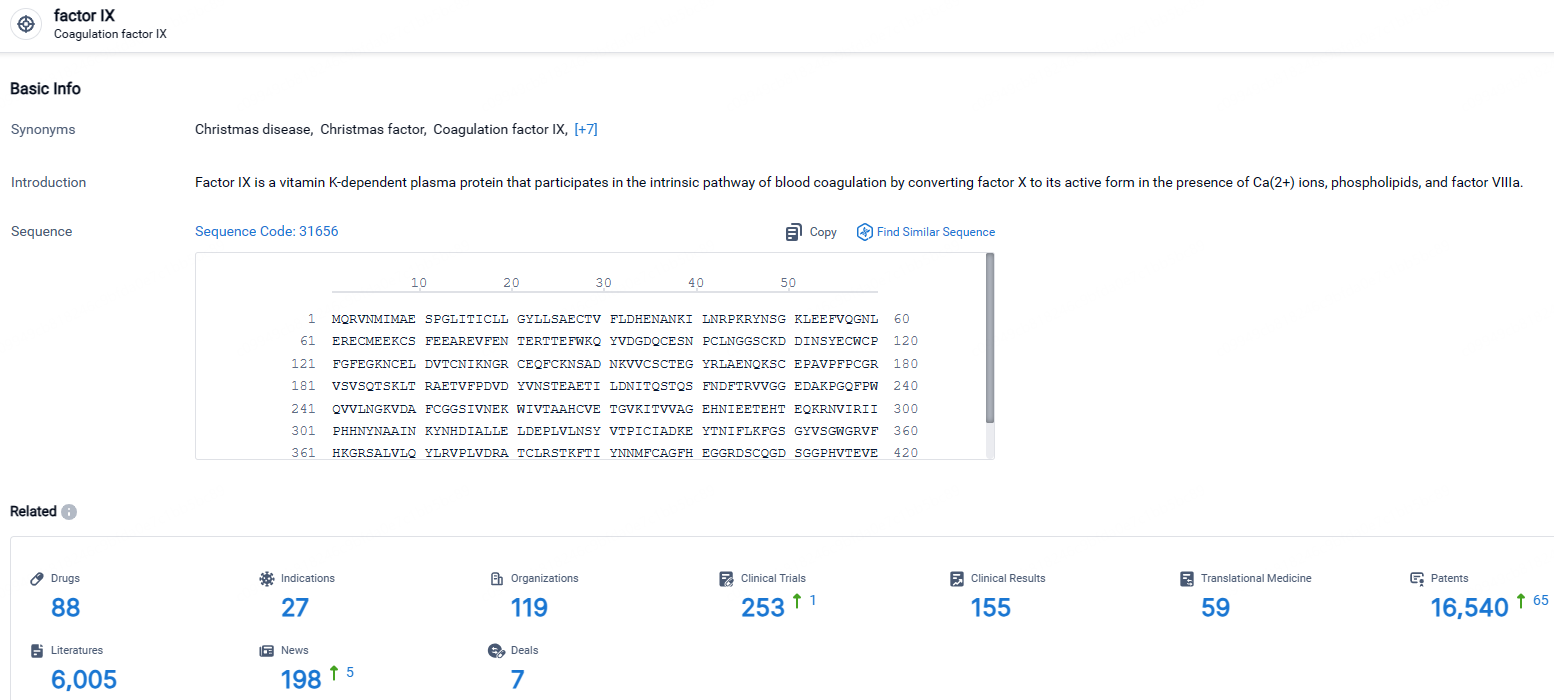
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 30, 2024, there are 88 investigational drugs for the FIX targets, including 27 indications, 119 R&D institutions involved, with related clinical trials reaching 253, and as many as 16540 patents.
fidanacogene elaparvovec-dzkt is an AAV-based gene therapy drug that targets factor IX. It falls under the therapeutic areas of Congenital Disorders and Hemic and Lymphatic Diseases, specifically for the treatment of Hemophilia B. The drug has been developed by The Children's Hospital of Philadelphia and has reached the highest phase of development, which is approval.