Testing Dosage of New T-cell Engager, ARB202, in Advanced Gastrointestinal Cancer Patients
Arbele, a biotechnology firm in the clinical phase that specializes in creating new immunotherapy treatments specifically for advanced gastrointestinal cancers, has received a favorable recommendation from the Data Monitoring Committee during its second meeting. The committee endorsed the continuation of the A001 Phase I clinical trial, which assesses the effectiveness of cabotamig (ARB202) in individuals battling advanced gastrointestinal cancers, without any changes to the study's protocol.
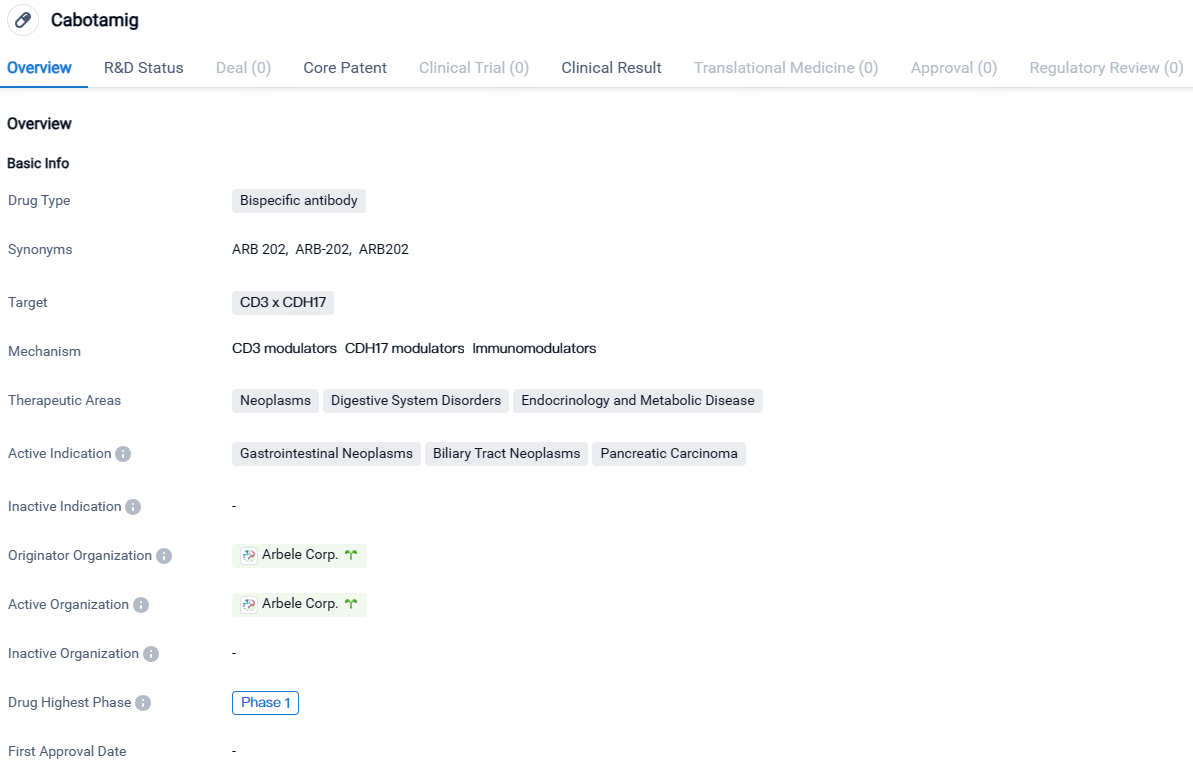
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This evaluation of safety was conducted through the analysis of data obtained from 18 individuals, including those who received several treatments with cabotamig. The favorable recommendation reaffirms that the tolerability of cabotamig aligns with the existing safety profiles for T-cell engagers used in different medical conditions.
"We owe a great deal of gratitude to the brave participants of this initial cabotamig study, which has enhanced our comprehension of its safety characteristics and helped refine the dosage for this hopeful treatment," stated Dr. Dennis Wong, the Chief Medical Officer at Arbele.
The A001 study is designed as a multi-center, open-label, First-in-Human (FIH) continuous trial. Its purpose is assessing the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and anti-tumor efficacy of cabotamig when delivered intravenously to patients with advanced GI malignancies unresponsive to conventional treatments and which express CDH17. The initial segment of this research includes a dose-escalation phase with about 28 participants, followed by a study where multiple doses are progressively increased or ranged.
Screening for tumors expressing CDH17 is mandatory for all participants except those with colorectal cancer, utilizing the TibTech™ automated diagnostic tissue platform. Patients exhibiting CDH17 will proceed to screening for dosage eligibility. Conversely, those with colorectal cancer do not require CDH17 marker tests for eligibility.
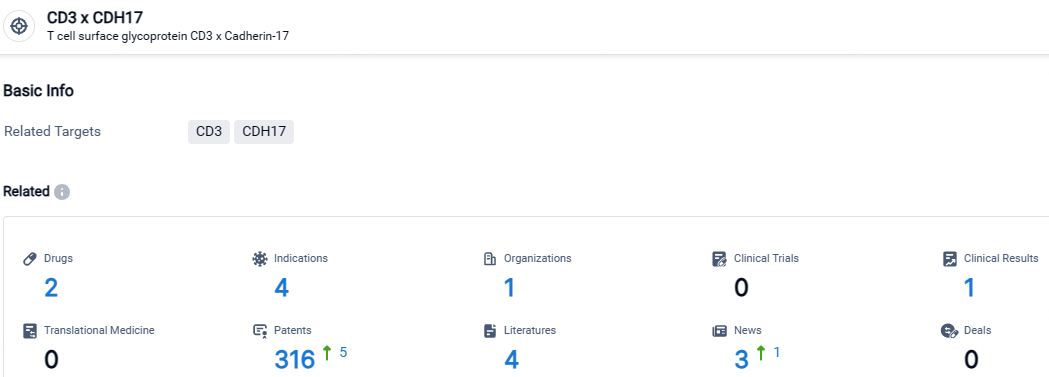
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 30, 2024, there are 2 investigational drugs for the CD3 and CDH17 target, including 4 indications, 1 R&D institutions involved, and as many as 316 patents.
Cabotamig shows promise as a potential treatment for gastrointestinal neoplasms, biliary tract neoplasms, and pancreatic carcinoma. However, further research and clinical trials are needed to assess its efficacy and safety in larger patient populations. The Phase 1 trial will provide valuable insights into the drug's potential and pave the way for further development and testing in subsequent phases.