FDA Approves Ractigen's RAG-01, a Novel saRNA Therapy for BCG-Resistant NMIBC
Ractigen Therapeutics, a pioneer in small activating RNA (saRNA) therapeutic development, has received approval from the U.S. Food and Drug Administration for its Investigational New Drug application concerning RAG-01. This innovative saRNA treatment is aimed at addressing non-muscle invasive bladder cancer. With this regulatory endorsement, Ractigen Therapeutics is poised to commence clinical trials in the United States, building on the foundation of a Phase I trial that began successfully in Australia.
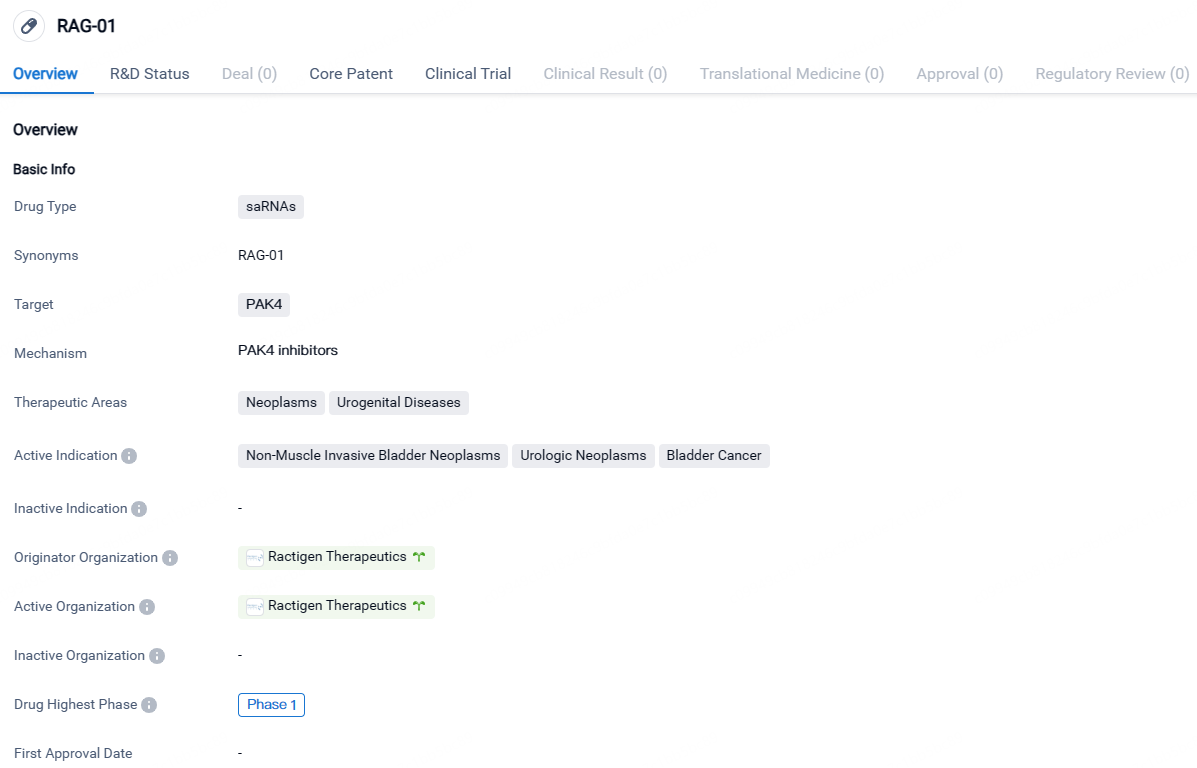
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
RAG-01 represents a groundbreaking treatment in the management of bladder cancer, marking a novel entry as it targets and activates the p21 tumor suppressor gene directly. This gene is crucial for regulating the progression of the cell cycle and plays an essential role in halting the proliferation of cancer cells. Activating p21, RAG-01 adopts a specific strategy that has the potential to slow the progression of NMIBC, an often encountered type of bladder cancer.
“Receiving FDA IND approval for RAG-01 is a pivotal moment for Ractigen and marks a substantial progress in saRNA technology globally,” commented Dr. Long-Cheng Li, the Founder and CEO of Ractigen Therapeutics. “As a trailblazing saRNA therapy, it utilizes the capability of RNAa to specifically target the p21 gene, providing a hopeful alternative for those with few treatment options. This approval underscores RAG-01’s potential as a frontrunner in saRNA therapy and cements our role as leaders in developing RNA-based therapies.”
RAG-01 is an innovative saRNA candidate designed to pinpoint and stimulate the tumor suppressor gene p21 through the RNAa mechanism. p21, often considered to be beyond the reach of traditional therapeutic interventions, offers a distinctive target for saRNA-enabled activation. Administered via intravesical instillation using Ractigen’s unique LiCO™ delivery system, RAG-01 has demonstrated considerable effects in reducing tumors in orthotopic bladder cancer models in mice.
The Phase I clinical trial of RAG-01 in Australia for NMIBC patients has already made impressive progress, with the initial three patients successfully enrolled and treated. The progress in the development of RAG-01 signifies a valuable advancement in RNAa-based therapeutic applications, aiming to meet the pressing needs of NMIBC patients.
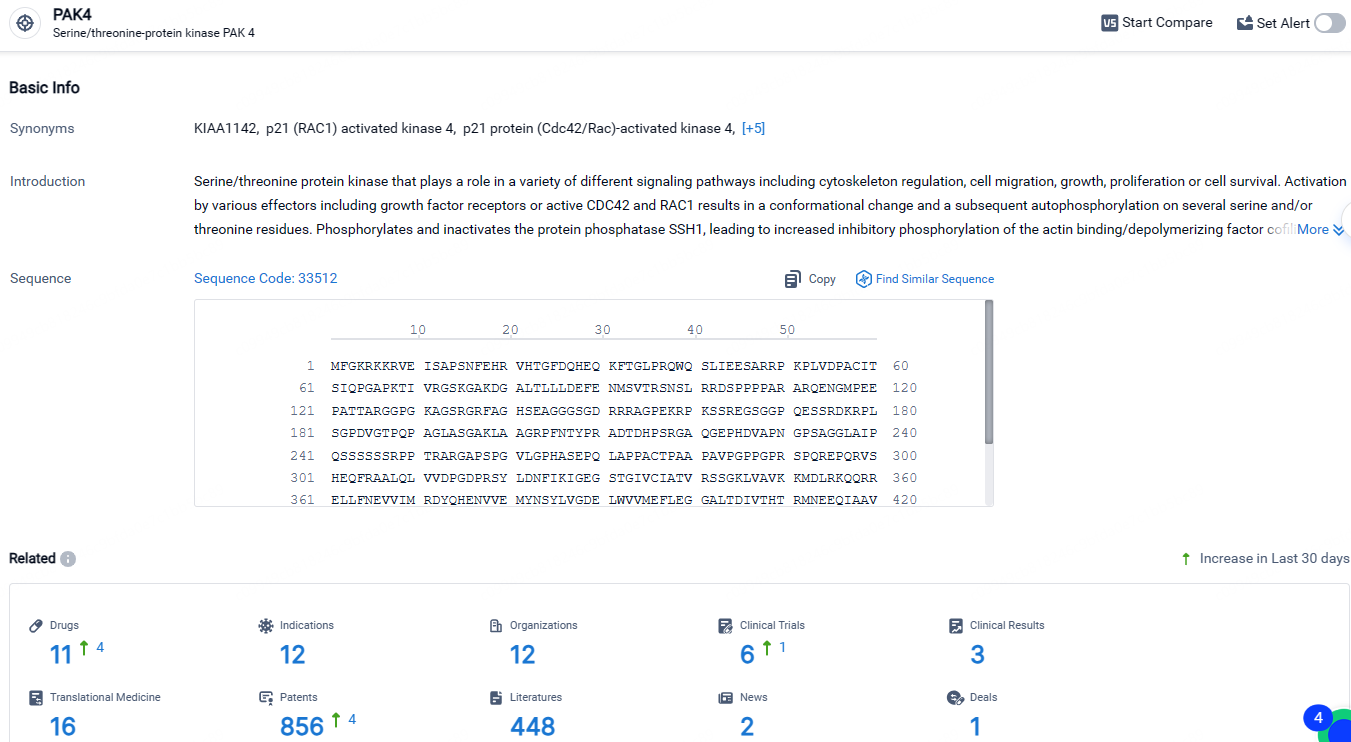
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 30, 2024, there are 11 investigational drugs for the PAK4 target, including 12 indications, 12 R&D institutions involved, with related clinical trials reaching 6, and as many as 856 patents.
RAG-01 is a saRNA drug developed by Ractigen Therapeutics, targeting the PAK4 protein. It shows potential for treating neoplasms and urogenital diseases, particularly non-muscle invasive bladder neoplasms, urologic neoplasms, and bladder cancer. While it has reached Phase 1 of development globally, it is still in the preclinical phase in China.