FDA approves Tarsus new drug Xdemvy for the treatment of demodex blepharitis
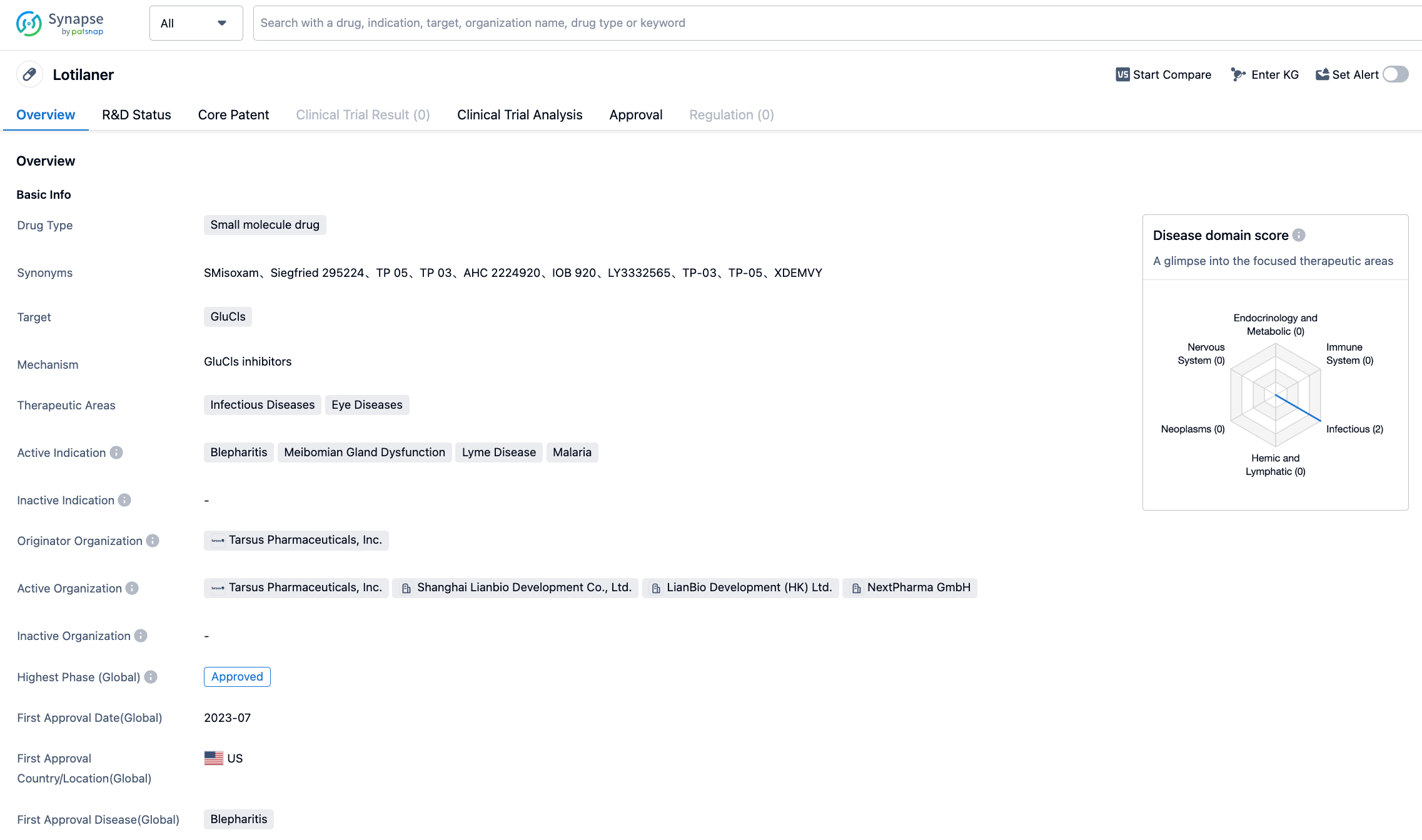
On July 26, 2023, Tarsus Pharmaceuticals announced that the U.S. FDA has approved Xdemvy (lotilaner ophthalmic solution, 0.25%) for the treatment of Demodex blepharitis. Xdemvy is a Lotilaner 0.25% ophthalmic solution, and it's the first and only FDA-approved treatment method that directly targets Demodex mites.
Previously known as TP-03, Xdemvy is a new prescription eye drop designed to treat Demodex blepharitis by targeting the root cause of the disease - Demodex infestations. The active ingredient in Xdemvy is lotilaner, a molecule that eradicates Demodex mites by selectively inhibiting the mite's GABA chloride channel, and is well-characterized. Due to its high lipophilicity, it facilitates its absorption in the oily sebum of the lash follicles where the mites reside.
Xdemvy was evaluated in two pivotal trials involving more than 800 patients. Both trials reached their primary endpoints and all secondary endpoints with statistical significance, and there were no serious treatment-related adverse events, with adverse reactions at 2%.
This FDA approval for the drug's marketing was also based on these two clinical trials. Patients with Demodex Blepharitis were randomly assigned to the Xdemvy group or solvent group in a 1:1 ratio and were dosed twice daily in each eye over 6 cycles.
In each trial, substantial therapeutic effects were observed by Day 43, with significant improvements in eyelids, and collarettes (wax hard shells) reduced to no more than 2 on each upper eyelid, with some patients observing improvements as early as the second week.
Moreover, by Day 43 in both trials, mite eradication endpoints (0 mites/eyelash density) and cured erythema (grade 0) also showed statistically significant improvement. Xdemvy generally shows a good safety and tolerability profile. The most common ocular adverse reactions observed in the study were stinging and burning at the instillation site, reported by 10% of the patients.
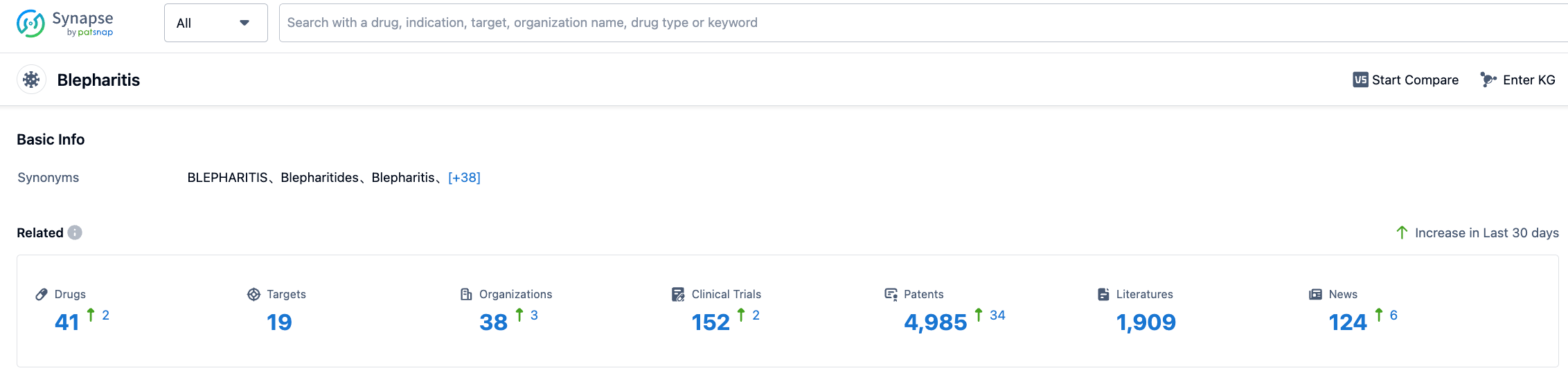
According to the information disclosed by Synapse (click on the card below to directly access the blepharitis indication. After registration and login, you can get free access to detailed information about the investigational drugs, targets, R&D institutions, clinical trials, etc. under this indication), as of July 27, 2023, there are a total of 41 investigational drugs under the blepharitis indication, including 19 types of targets. There are 38 research institutions and 152 related clinical trials, and 4964 patents... The successful approval of this new drug provides a new treatment option for patients in the United States.