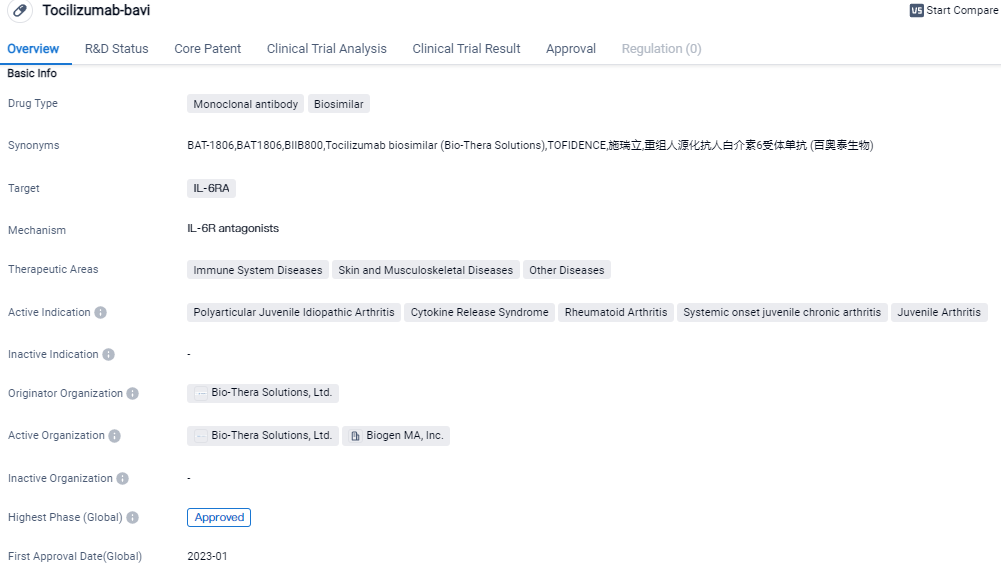
FDA Grants Authorization for Biogen's TOFIDENCE™ (tocilizumab-bavi), a Biosimilar Linked to ACTEMRA®
Biogen Inc. disclosed that the U.S. FDA provided their endorsement for TOFIDENCE (tocilizumab-bavi) in an intravenous format, a biosimilar monoclonal antibody that references ACTEMRA. It's been authorized for combating conditions like moderately to severely active rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and systemic juvenile idiopathic arthritis, as part of the TOFIDENCE intravenous formulation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TOFIDENCE is the premier tocilizumab biosimilar to receive approval in the US. Biosimilars are biologic goods proven to provide similar effectiveness and safety to the approved reference product. They hold a significant advantage, as they can result in cost savings and allow for broader, long-term access to treatments.
The past decade has seen consistent year-on-year rises of 10%–25% in autoimmune disease therapy expenditures. Since the introduction of biosimilars in the U.S market, medicines facing biosimilar competition have seen increased patient acceptance, equating to over 150 million days of patient therapy.
Ian Henshaw, Global Head of Biosimilars at Biogen, commented on the approval of TOFIDENCE in the United States, stating that it serves as a positive progression towards enhancing access to key treatments for more people suffering from chronic autoimmune conditions. He added that an increase in approved biosimilars would see potential growth in savings for healthcare systems, more choice for physicians, and wider access to biologics for patients.
Biogen formed a commercialization and licensing agreement with Bio-Thera for TOFIDENCE in April 2021. Bio-Thera, the developers of TOFIDENCE, have handed the commercialization rights in the United States to Biogen. According to this agreement, Biogen holds exclusive privileges regarding the regulation, production, and marketing of TOFIDENCE in all nations excluding China. Biogen is in the process of assessing the prospective launch timeline for TOFIDENCE in the U.S.
Biogen's FDA approval of TOFIDENCE was granted following a thorough submission of analytical, non-clinical, and clinical data to the FDA in September 2022. A rigorous analytical characterization of the structural, physicochemical, and biological features of TOFIDENCE has been undertaken, backing up its biosimilarity with the reference product.
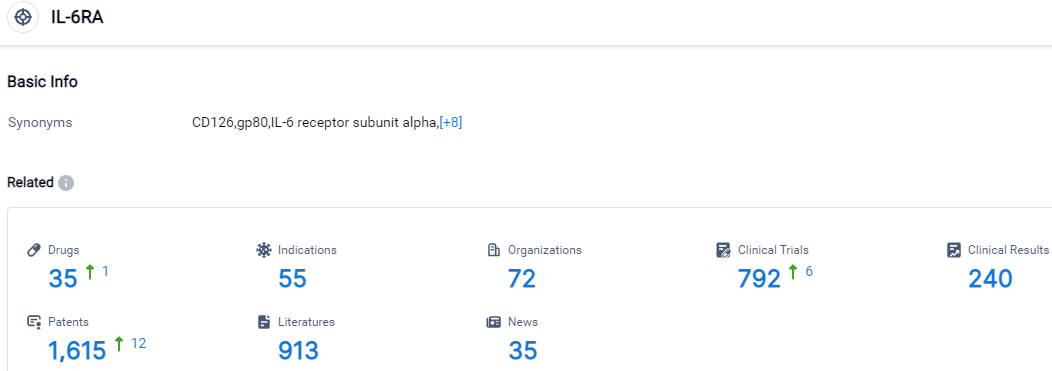
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 9, 2023, there are 35 investigational drugs for the IL-6RA target, including 55 indications, 72 R&D institutions involved, with related clinical trials reaching 792,and as many as 1615 patents.
TOFIDENCE (tocilizumab), is a treatment developed as a biosimilar to the reference product ACTEMRA. TOFIDENCE is indicated for the treatment of moderately to severely active rheumatoid arthritis, polyarticular juvenile idiopathic arthritis and systemic juvenile idiopathic arthritis.