FDA to Hold Panel Review for Examination of the TRAILBLAZER-ALZ 2 Trial Results on Donanemab
Eli Lilly and Company has revealed that the U.S. Food and Drug Administration is planning to assemble a session of the Peripheral and Central Nervous System Drugs Advisory Committee. This gathering will focus on deliberations concerning the Phase 3 TRAILBLAZER-ALZ 2 study outcomes, which have been examining the effectiveness and safety of the investigational treatment donanemab in patients with the early symptomatic stages of Alzheimer's disease.
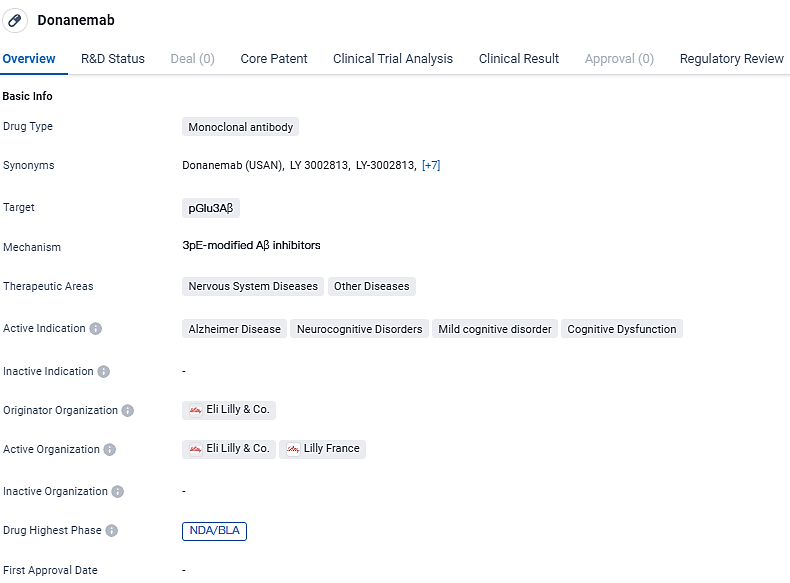
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The FDA has communicated with Lilly expressing a need to delve deeper into issues surrounding the assessment of donanemab's risk profile and the potential benefits it offers. This includes examining the safety findings in patients who were administered donanemab and analyzing the implications of the distinct methodologies employed in the TRAILBLAZER-ALZ 2 study. The study's design features a finite dosing schedule, permitting the conclusion of treatment based on evaluating amyloid plaque levels, and it also considers the inclusion of subjects with specific tau protein concentrations.
There is no confirmed schedule for the FDA's advisory committee meeting to discuss donanemab, leading to an anticipation that the regulatory decision on this therapy will extend past the initial phase of 2024. Advisory committee meetings taking place after the expected date of FDA decision is atypical, yet for donanemab, the process aligns with the precedent set by prior advisory discussions for other approved therapies targeting amyloid plaques.
Anne White, Lilly's executive vice president and president of Lilly Neuroscience, commented, "The decision by the FDA to organize an advisory committee review at this juncture was unforeseen. Nevertheless, we are prepared to engage in a thorough presentation of the TRAILBLAZER-ALZ 2 study outcomes, highlighting donanemab's potent efficacy within a safety framework. Our aim is to collaborate closely with the FDA and community stakeholders during this presentation to address any inquiries."
In contrast to other studies on comparable amyloid plaque-targeting treatments, TRAILBLAZER-ALZ 2 had subjects who were further along in the progression of their condition. Benefits from donanemab treatment were seen across all participant groups, irrespective of their tau protein levels, with those at earlier stages displaying the most significant improvements.
The clinical advantages of donanemab were evident even with a short-duration treatment protocol, as almost half the study's participants finished their therapy within a span of six to twelve months. The primary safety concern linked to donanemab use is the potential for amyloid-related imaging abnormalities (ARIA), which pose severe and potentially fatal risks. Other common side effects noted include reactions related to the infusion, headaches, and queasiness.
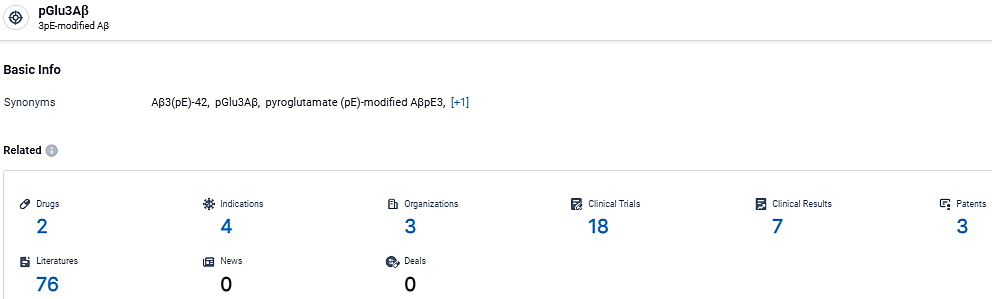
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 2 investigational drugs for the pGlu3Aβ target, including 4 indications,3 R&D institutions involved, with related clinical trials reaching 18, and as many as 3 patents.
Donanemab targets pGlu3Aβ and is indicated for the treatment of Alzheimer's Disease and other neurocognitive disorders. The drug has reached the highest phase of NDA/BLA globally and in China, and has received regulatory designations of Priority Review and Breakthrough Therapy. Further research and regulatory approval are needed to determine the full potential of Donanemab in addressing the challenges posed by neurocognitive disorders.