Progress on Amgen's Weight Loss Pipeline: AMG 133
In February, the Amgen team published an article in the journal Nature Metabolism, reporting the potential of the company's novel bifunctional molecule AMG 133 in treating obesity.
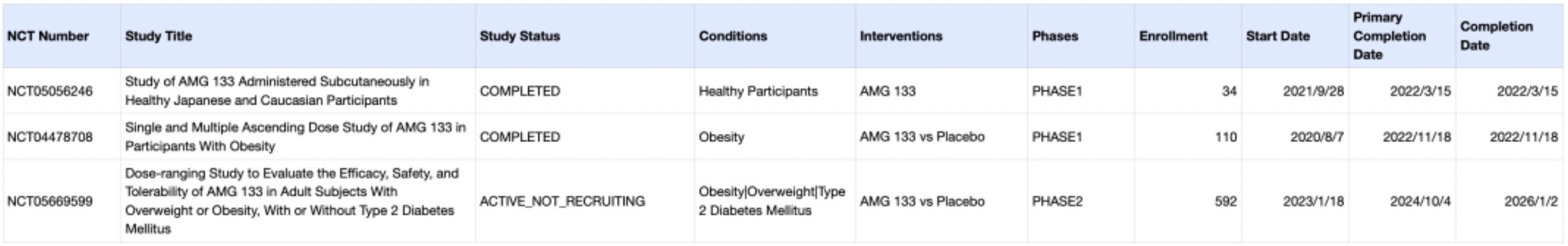
It is reported that the molecule is designed by conjugating a fully humanized anti-GIP receptor monoclonal antibody with a GLP-1 analog peptide segment via an amino acid linker, forming a drug candidate with a dual-action mechanism. In a Phase 1 randomized, double-blind, placebo-controlled trial involving obese adults (NCT04478708), AMG 133 exhibited good safety and tolerability profiles and demonstrated significant dose-dependent weight loss effects.
The molecule is currently undergoing a Phase 2 clinical study for obesity and type 2 diabetes (NCT05669599), with research findings expected to be updated by the end of 2024.
About Murielle M. Véniant
Murielle M. Véniant is the Executive Director of the Research and Development department at Amgen, focusing on early drug development for cardiovascular and metabolic diseases. Currently, she leads a team in Amgen's Cardiovascular and Metabolic Diseases Discovery Research division, dedicated to the creation of new drugs for conditions such as obesity and heart failure.
Since 2000, she has been a key member of the Amgen cardiovascular and metabolic disorders research team, guiding both large- and small-molecule candidate drugs from basic science research to phase I clinical trials. Murielle holds a PhD in Pharmacology from the University of Dijon in France, in collaboration with the Roche company in Switzerland. Her academic background, coupled with extensive practical experience, has made her highly effective in the field of translational medicine, significantly advancing the translation of scientific findings in cardiovascular and metabolic diseases into clinical applications.
Throughout her career, Murielle has played an instrumental role in the development journey at Amgen. She initially led the promotion of diabetes projects, particularly in creating and expanding the in vivo pharmacology research team at the Thousand Oaks laboratory. In 2011, she successfully integrated the research capabilities from San Francisco, greatly enhancing Amgen's competitive edge in the treatment of diabetes.
Over the past two decades, Murielle has made a series of highly representative research achievements in the field of cardiovascular and metabolic diseases.
Currently, Murielle and her team are focused on the clinical development of the novel multispecific molecule AMG 133. This strategy is based on an in-depth understanding of the distinct mechanisms of action of the two incretin hormones, GIP and GLP-1, in regulating body weight, glycemic control, and fat storage. Through preclinical and phase I clinical trials, AMG 133 has demonstrated significant weight reduction and improvement in various metabolic parameters, paving a new path for the treatment of obesity and other related metabolic diseases.
Drug Discovery of AMG 133
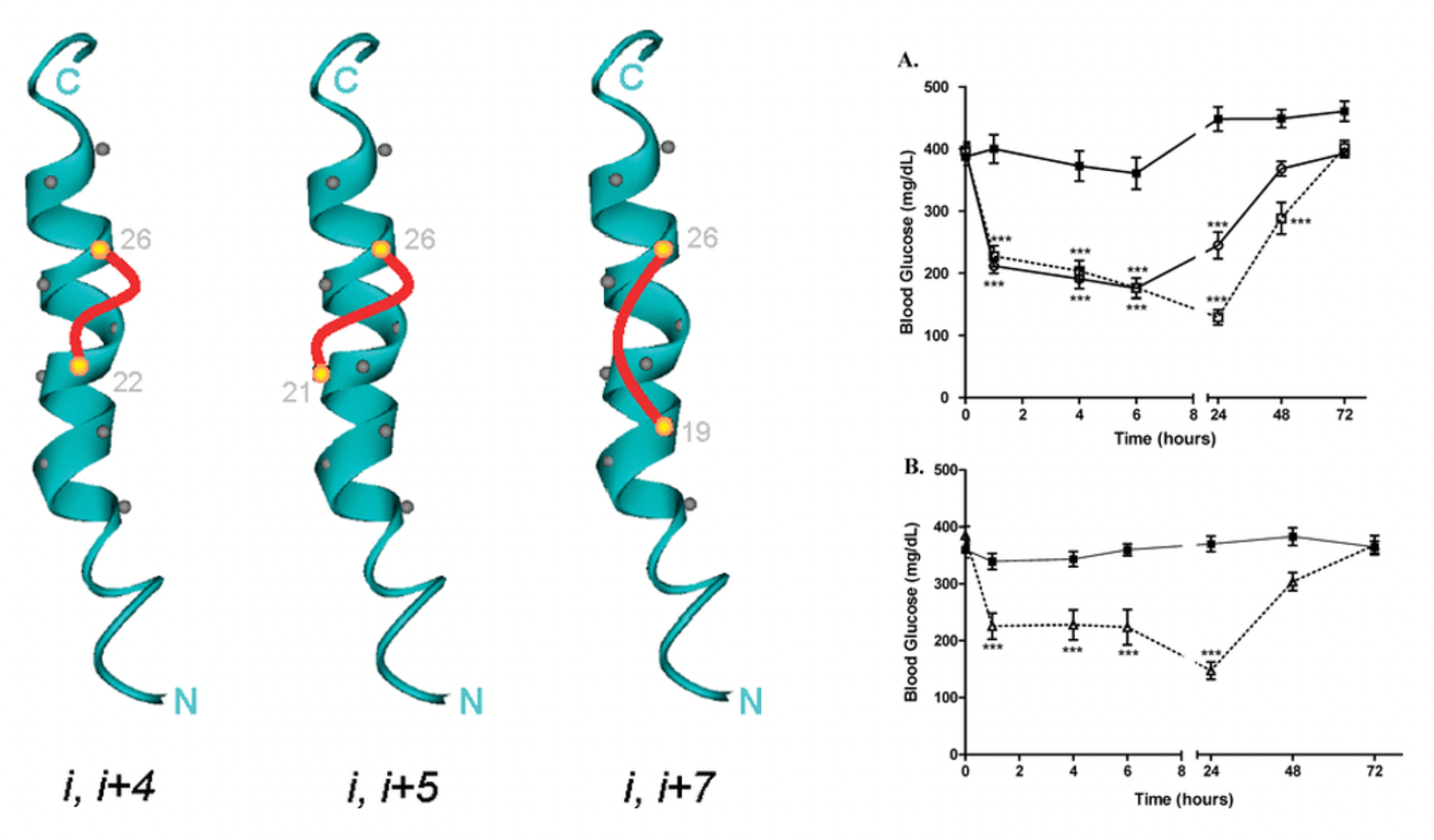
The first research outcome of Murielle's team on the GLP-1R related project was published in the journal "J Med Chem" in 2008. In this study, a series of long-acting GLP-1 derived peptides were designed and synthesized. The PEGylated peptides (23) and (24) retained the functional potency of native GLP-1 and its receptor binding characteristics, with (23) demonstrating the ability to lower blood glucose levels and reduce body weight over several days in non-human primates.
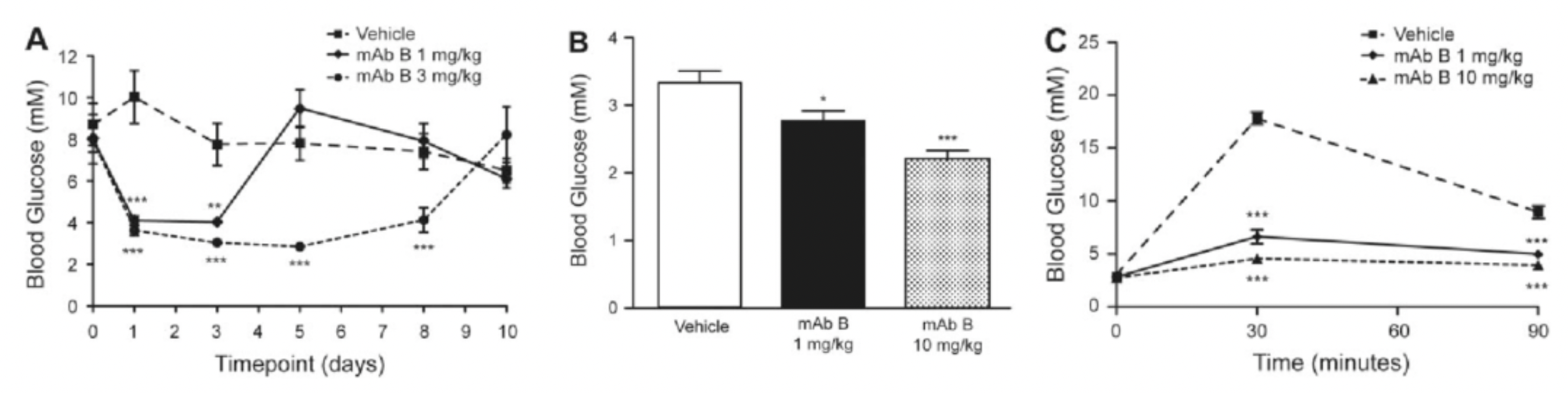
Subsequently, Murielle's team developed a high-affinity monoclonal antibody targeting the human glucagon receptor (GCGR). This antibody exhibited potent antagonistic activity both in vitro and in vivo. A single injection of 3 mg/kg of this antibody normalized blood glucose levels in obese mice over 8 days. In addition, a single injection of the antibody decreased fasting blood glucose levels in a dose-dependent manner in normal C57BL/6 mice without inducing hypoglycemia, and improved glucose tolerance.
At the end of 2016, the Amgen team filed a patent (US20170275370A1) titled "Methods for Treating or Ameliorating Metabolic Disorders Using a GIPR Binding Protein in Combination with a GLP-1 Agonist." This reported the therapeutic potential of using an antibody targeting the GIPR in combination with a GLP-1 receptor agonist for the treatment of type 2 diabetes, obesity, dyslipidemia, elevated levels of glucose, increased insulin levels, and diabetic nephropathy.
The patent describes a method of nucleic acid immunization used by the Amgen team to stimulate a specific immune response against mouse GIPR (Gastric Inhibitory Polypeptide Receptor) in mice. Subsequently, specific antibodies were obtained using classical hybridoma antibody screening techniques. Initially, the hybridoma antibodies belonged to the mouse IgG2a subtype. To reduce the effector functions mediated by the Fc fragment, researchers converted the antibody subtype to a non-glycosylated mouse IgG1 subtype by introducing an N297 to G mutation to achieve deglycosylation.
In the development process of producing humanized antibodies against human GIPR, XENOMOUSE® transgenic mice were used, combined with nucleic acid immunization and immunization with the soluble N-terminal structural domain (1-139) protein antigen of human GIPR.
Using the mouse anti-GIPR antibody 2.63.1, researchers found that the antibody not only antagonized the insulin secretion induced by GIP but also exhibited a certain effect on weight reduction. This suggests that the antibody may exert a positive influence on energy metabolism and weight regulation by affecting the GIP signaling pathway, potentially achieved by reducing food intake, inhibiting fat storage, or increasing energy expenditure, etc. These data indicate that the anti-GIPR antibody 2.63.1, as a potential therapeutic approach for obesity, has demonstrated certain weight-reducing effects in animal models.
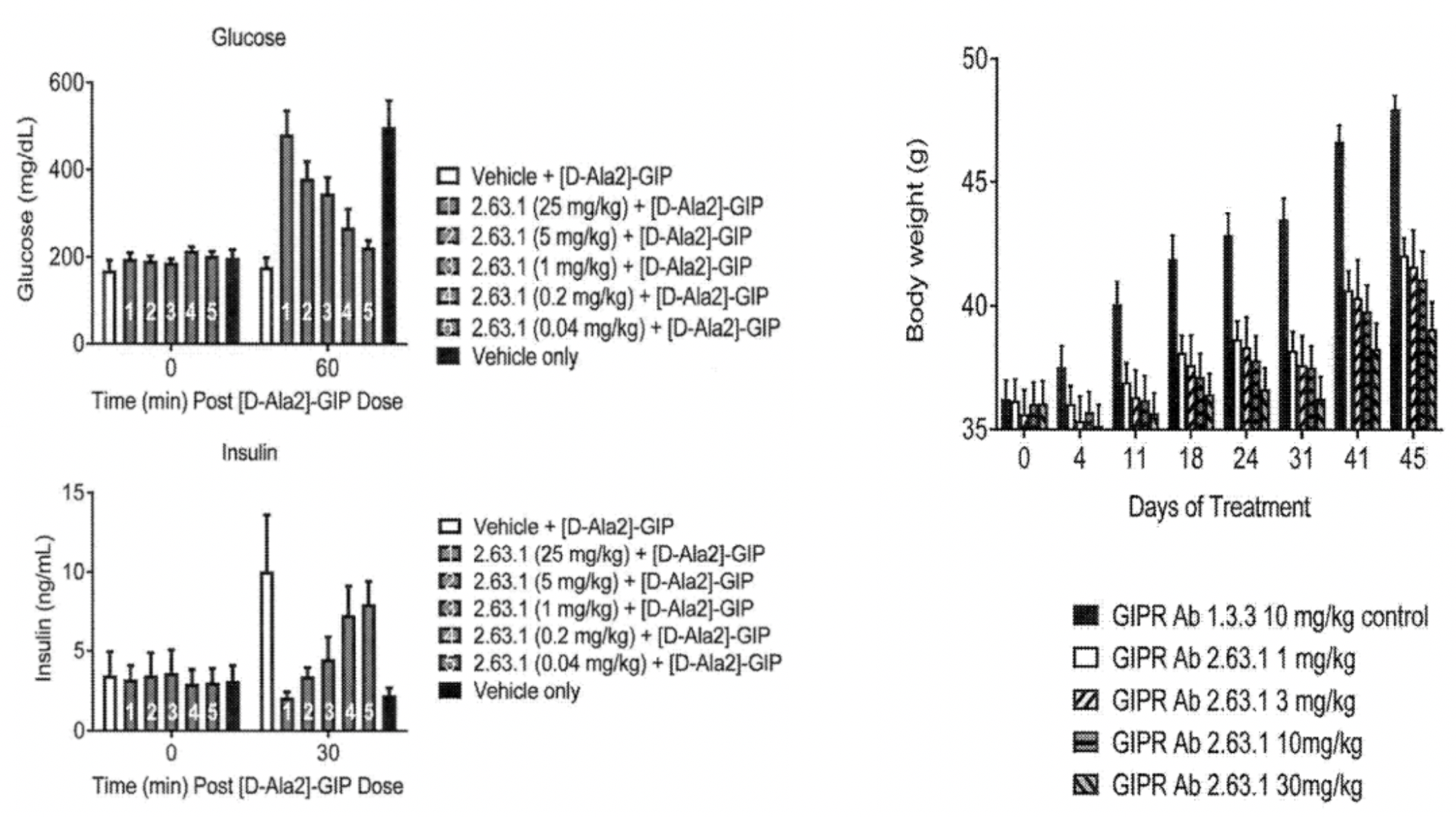
Furthermore, the antibody also demonstrated an effect of reducing fat mass (including both subcutaneous and visceral fat) without affecting lean body mass (primarily composed of muscle, bone, organs, blood, water, and other non-fat tissues).

Further research found that after treatment with the anti-mouse GIPR antibody 2.63.1, hepatocellular microvesicular changes were reduced, and the associated lipid accumulation also declined; a decrease in both the liver weight and hepatic triglyceride content in experimental animals was observed, thereby reconfirming the antibody's significant inhibitory effect on hepatic fat accumulation; inflammation in the peri-epididymal white adipose tissue of the experimental animals was alleviated, and the number of infiltrating macrophages was reduced, suggesting that the antibody could improve the inflammatory status of adipose tissue. This holds significant implications for the prevention and treatment of obesity and its related complications such as non-alcoholic fatty liver disease and type 2 diabetes.
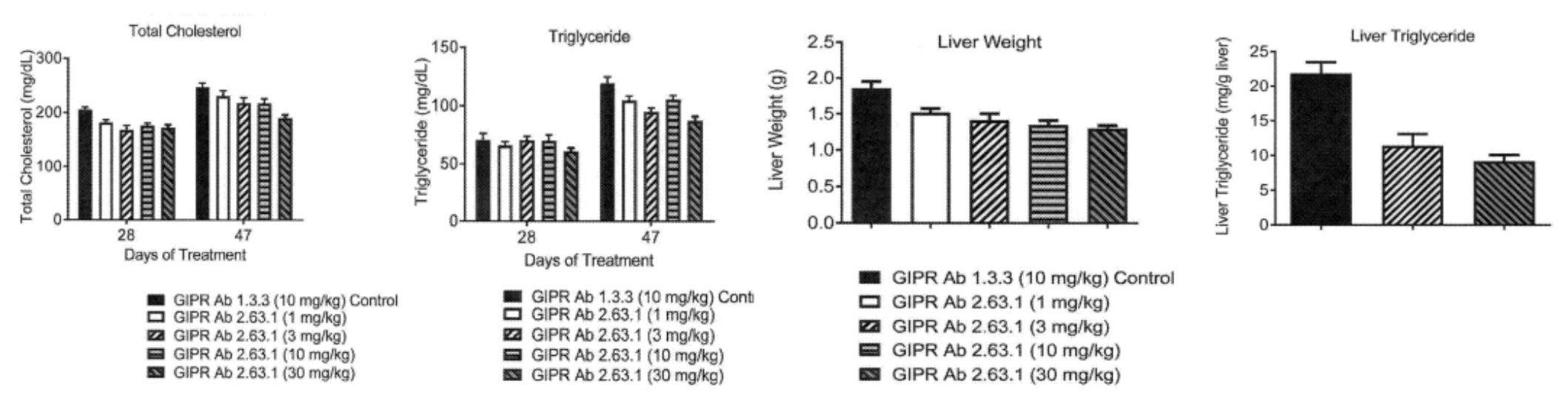
In 2018, the team led by Murielle published an article in the journal "Science Translational Medicine," reporting on the development of an anti-GIPR (Glucose-dependent Insulinotropic Polypeptide Receptor) antagonist antibody as a potential therapeutic strategy for treating obesity. The article described how the administration of an anti-mouse GIPR antibody (muGIPR-Ab) prevented weight gain in diet-induced obesity (DIO) mice, improved various metabolic parameters, and was associated with a reduction in food intake and the resting respiratory exchange ratio. The use of an anti-human GIPR antibody (hGIPR-Ab) replicated these results in obese non-human primates (NHPs), with an even more pronounced weight loss observed than in mice. Furthermore, researchers observed that when the anti-GIPR antibody was used in combination with a glucagon-like peptide-1 receptor agonist, the weight loss effects were enhanced in both DIO mice and non-human primates. These findings provide preclinical validation for the use of anti-GIPR antibodies in the treatment of obesity.
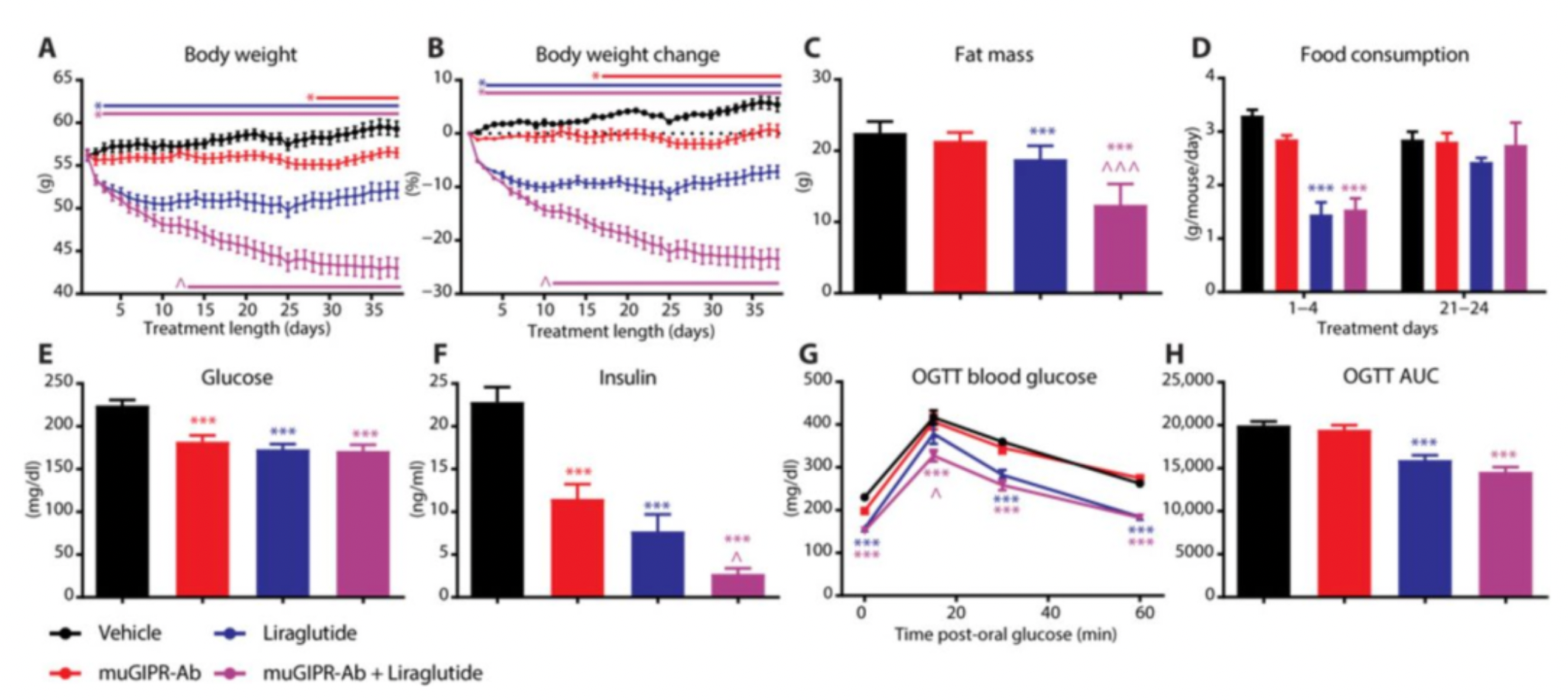
In 2018, the Amgen team updated a new invention patent titled "Methods for Treating or Ameliorating Metabolic Disorders Using a Gastric Inhibitory Polypeptide Receptor Antagonist Bound to a Glucagon-Like Peptide-1 Receptor Agonist Fusion Protein (WO2018237095A1)." In this patent, researchers have created a fusion by linking the carboxyl-terminal end of a GLP-1 analog to the amino-terminal end of either the light chain variable region or the heavy chain variable region of an antibody or its functional fragment capable of binding to the GIPR receptor. A linker may also be included between the two components. This design implies the combination of a physiologically active GLP-1 analog and a specific antibody targeting GIPR, forming a dual-functional molecule. This fusion protein is capable of exerting the beneficial effects of GLP-1 on blood glucose regulation and insulin secretion while also targeting and inhibiting GIPR function through the antibody fragment. Thereby, it may achieve more precise and effective treatment outcomes in the therapy of metabolic diseases such as type 2 diabetes and obesity. The role of the linker is to ensure that the two different protein structural domains are correctly connected and maintain their biological activity without degradation.
Based on the implementation example data provided by the patent, researchers conducted extensive optimization of peptides, antibodies, and linker peptides. Optimization was performed by comparing the expression levels of fusion proteins and evaluating their agonistic activity towards GLP-1R, antagonistic activity towards GIPR, pharmacokinetic properties, as well as changes in body weight and blood glucose levels.
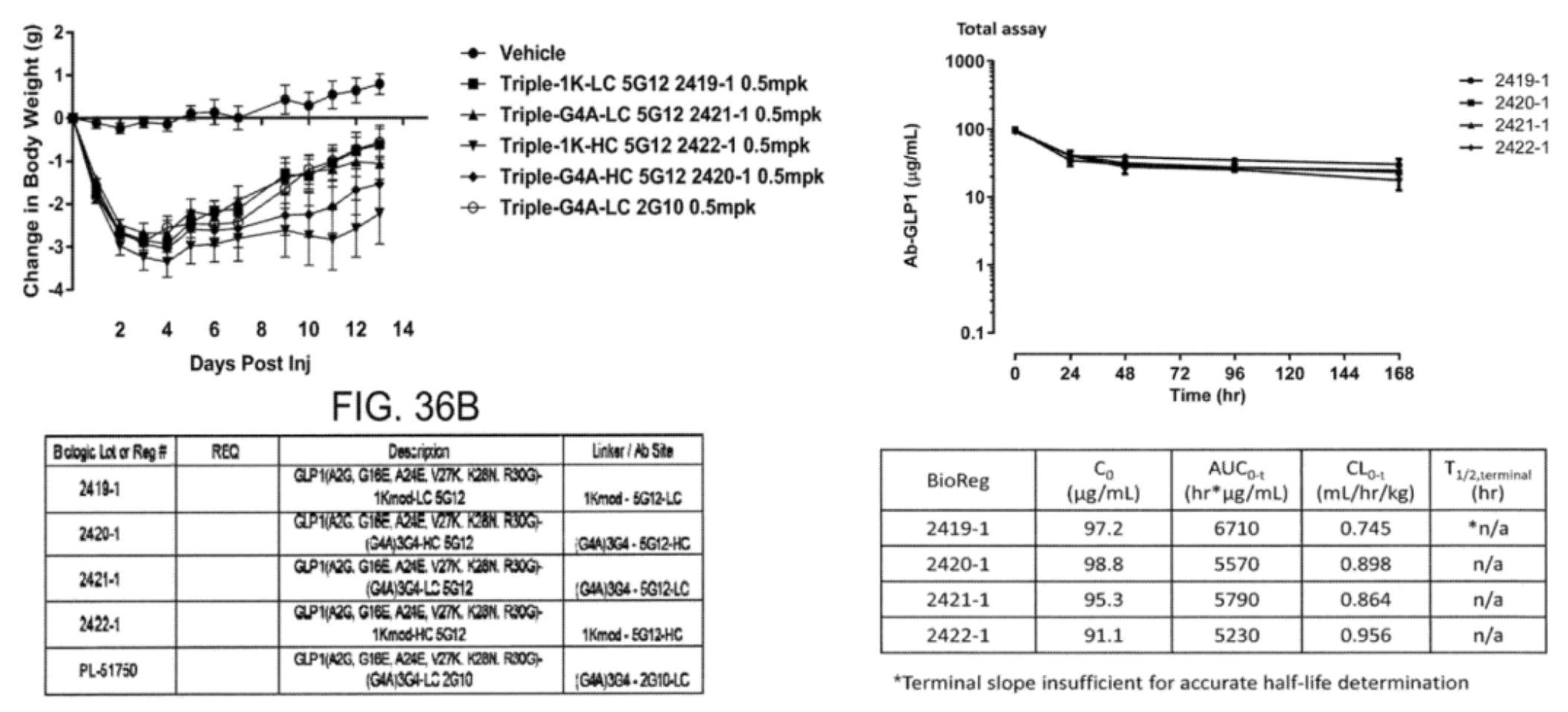
In 2021, the Amgen team published the preclinical research progress of their dual-function molecule project AMG 133, which involves GIPR antibodies and GLP-1R, for the first time in "Cell Reports Medicine".
The researchers chemically conjugated GLP-1 peptide segments containing a GGGGS tripeptide linker (such as P1 and P2) to GIPR antibodies through covalent bonds. The design of the P1 peptide enhances metabolic stability and in vivo persistence by incorporating 2-aminobutyric acid and replacing the glycine at position 36 of arginine. Meanwhile, the P2 peptide, through a D15E mutation, reduces the potency of GLP-1 activity, aimed at minimizing potential gastrointestinal side effects.
Both GIPR-Ab/P1 and GIPR-Ab/P2 maintained robust GIPR antagonistic actions on both human and monkey-derived GIPRs, with IC50 values comparable to the original GIPR antibody, indicating that chemical conjugation did not diminish the GIPR antagonistic activity. Concerning GLP-1R agonistic activity, all GIPR-Ab molecules containing GLP-1 fragments exhibited around 20 to 40 times lower efficacy in stimulating cAMP production compared to the natural GLP-1(7-37).
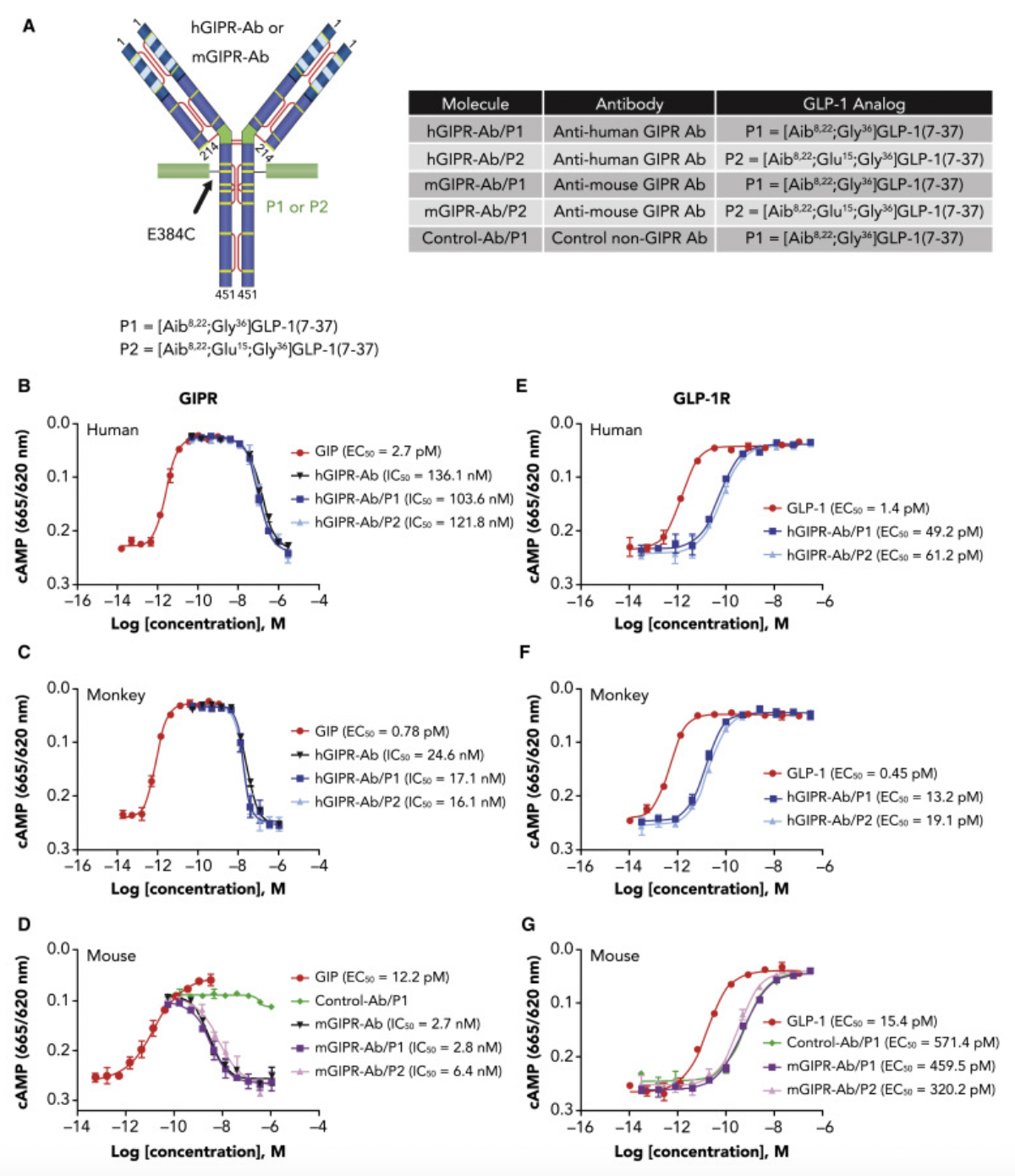
In animal models, these GIPR-Ab/GLP-1 molecules have shown significant effects in reducing body weight in obese mice and monkeys, as well as improving various metabolic parameters. Notably, compared to the use of GIPR antibodies alone or control antibody-GLP-1 conjugates, GIPR-Ab/GLP-1 exhibits a more potent weight-reducing effect, suggesting a synergistic interaction between the two targeting mechanisms. Additionally, this bifunctional molecule also leads to rapid internalization of the receptor, thereby amplifying the cAMP signal within intracellular vesicles. This phenomenon may explain its superior therapeutic efficacy in the treatment of obesity and related metabolic disorders.
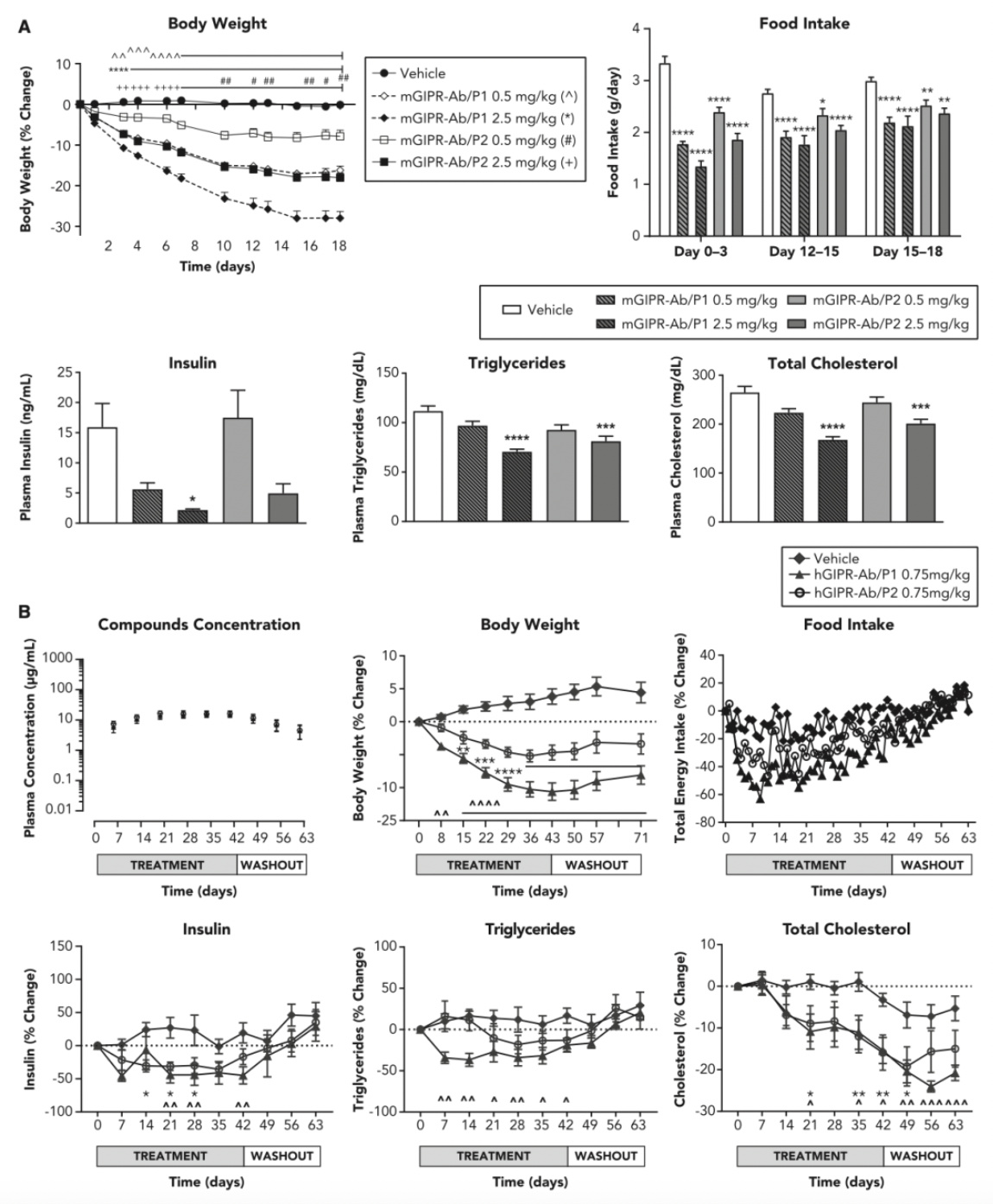
On February 5, 2024, the Amgen team published an article in the journal "Nat Metab," confirming the antagonistic activity of AMG 133 on GIPR and the agonistic activity on GLP-1R in cellular systems. The paper also reported the capability of AMG 133 to reduce body weight and improve metabolic indices in male obese mice and in monkeys.
Furthermore, in a Phase 1 randomized, double-blind, placebo-controlled clinical study (NCT04478708) in patients with obesity, adult obese subjects received either a single ascending dose (SAD) or multiple ascending doses (MAD) of AMG 133, compared with a placebo group. AMG 133 was shown to have acceptable safety and tolerability, and it significantly reduced body weight.
Clinical data indicated that the population receiving AMG 133 treatment exhibited a clear dose-dependent weight reduction. Specifically, with a dosage of 140 mg every four weeks, the average percentage change in body weight was a decrease of 2.0% on day 7, and by day 78, after three doses, there was an average weight reduction of 7.4%. With the highest dosage of 420 mg every four weeks, the effects were even more prominent: a weight reduction of 4.9% by day 7, escalating to 14.5% by day 85. In contrast, the placebo control group showed minor weight changes, with an average change of only 0.04% by day 7 and an increase of 1.5% by day 85. As the dosage of the drug increased, the patients' weight continued to decrease, and this reduction was maintained for a significant duration after the last administration, lasting up to 150 days.
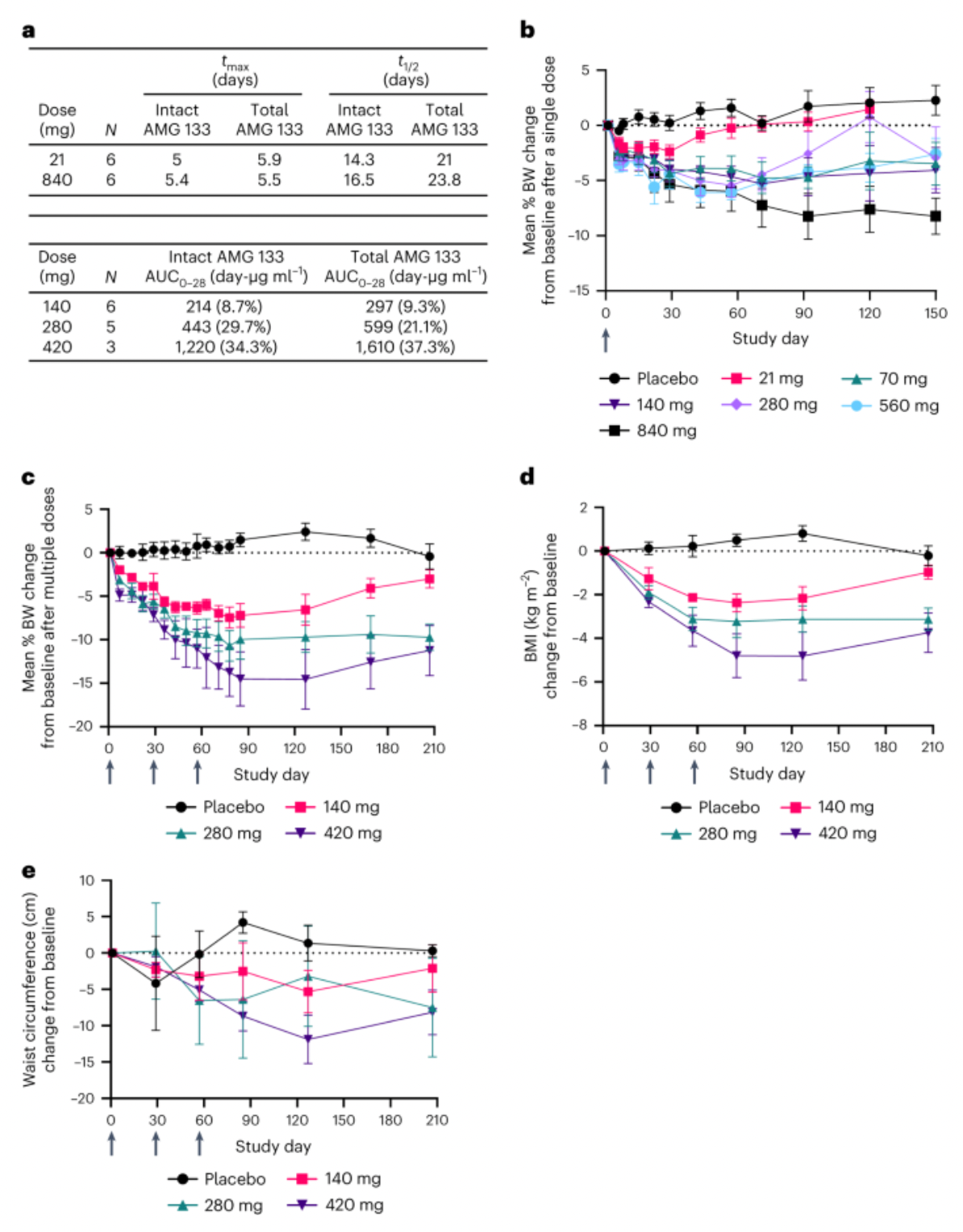
In the market, a large number of teams are developing therapeutic drugs targeting GLP-1R, GIPR, and GCGR. The Amgen team has chosen a less traveled path. There is considerable debate within the industry, particularly on whether to develop agonist or antagonist drugs targeting GIPR. The review article written by Murielle's team is well worth reading.
Ultimately, whether AMG 133 can overcome the challenges to achieve success, let us wait and see.