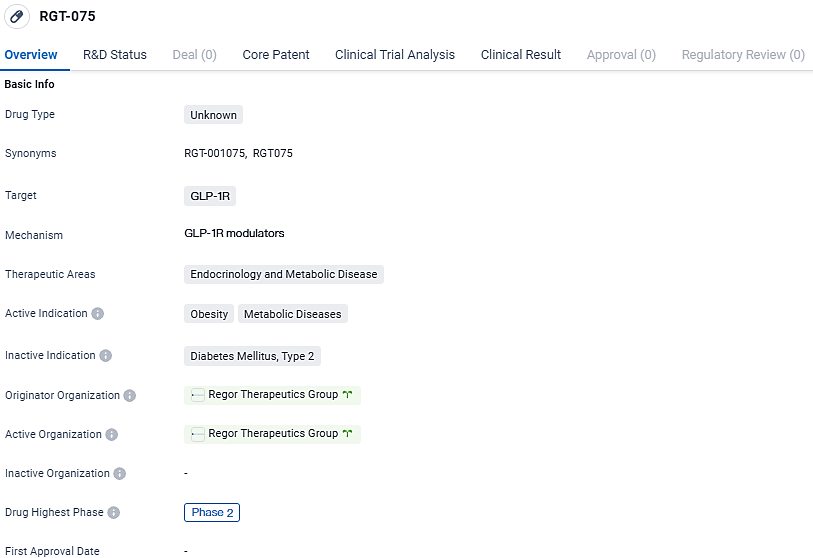
Regor Begins Second Stage Trial for Daily Oral GLP-1 Receptor Activator RGT-075 to Combat Excess Weight
Regor Therapeutics Group, an international enterprise operating in the clinical phase and specializing in biopharmaceuticals, supported by advanced drug research technology and a unique pipeline for clinical trials, has declared the commencement of its Phase 2 study. This study focuses on RGT-075, an orally administered, highly selective agonist of the GLP-1 receptor. The trial targets adult participants suffering from obesity or those overweight with associated secondary health conditions.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The preliminary findings for the ongoing Phase 2 clinical study are anticipated to be released towards the latter part of 2024. "Commencing the Phase 2 study is a significant benchmark in progressing RGT-075, a therapy we have confidence in potentially being a game-changer and more user-friendly option for individuals seeking treatments for obesity compared to existing options," stated Michael Grimm, M.D., Ph.D., the lead of the Metabolic Diseases program at Regor.
"RGT-075 has been formulated for once-a-day oral administration, either as a standalone therapy or in combination with other oral agents, aiming to manage obesity. The Phase 1 trial provided encouraging preliminary results on weight reduction even over a brief period of administration," continued Dr. Grimm.
This Phase 2 study, a multi-site, controlled, double-blind, and placebo-based clinical trial, is structured to assess the efficacy, safety, tolerable dosage limits, and the biological and physiological impacts of the daily oral use of RGT-075 in comparison to a placebo amongst overweight or obese adults with additional weight-related health concerns.
The study plans to include roughly 60 adults with a body mass index (BMI) ranging between 27 kg/m^2 and 45 kg/m^2, who will be assigned randomly to receive RGT-075 or a placebo. The intervention period spans 12 weeks. Participants receiving the RGT001-075 compound will undergo a 6-week titration period to the target dose, which will then be maintained for the remaining 6 weeks. It's expected that the preliminary data from this Phase 2 trial will be disseminated in the second half of 2024.
Regor has been pioneering the development of RGT-075, a once-daily, orally available small molecule that acts as a full agonist of the GLP-1 receptor, crafted for addressing metabolic disorders like type-2 diabetes and obesity. The compound has already progressed through Phase 1 single and multiple dose-escalation studies, demonstrating favorable safety profiles and tolerability.
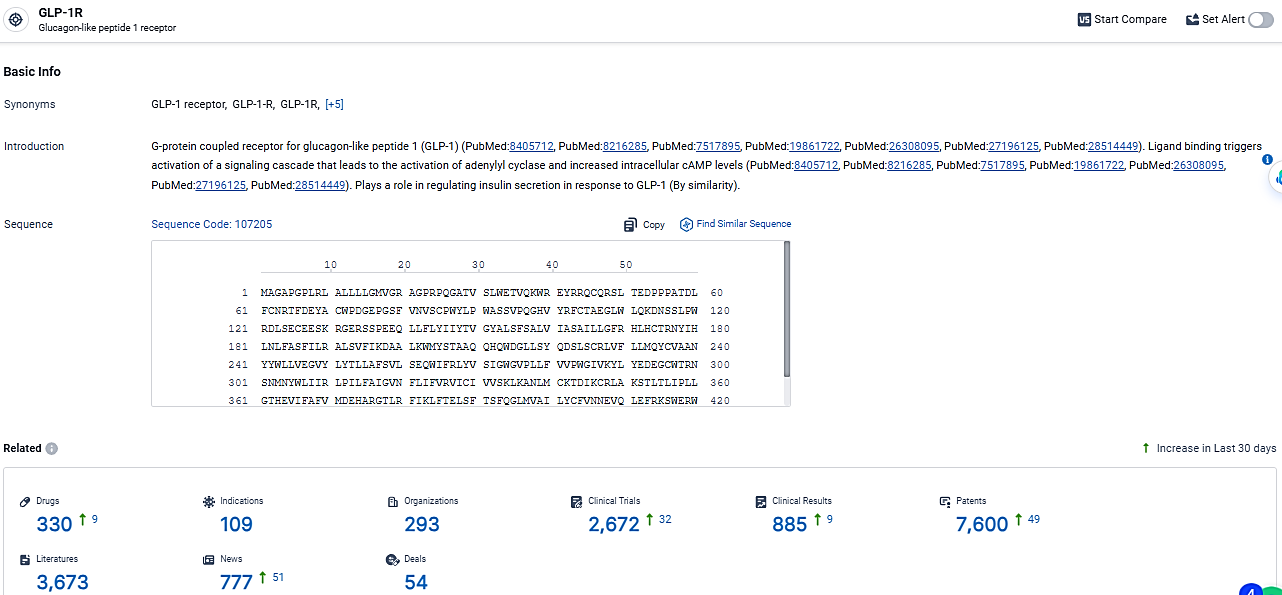
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 330 investigational drugs for the GLP-1R target, including 109 indications, 293 R&D institutions involved, with related clinical trials reaching 2672, and as many as 7600 patents.
RGT-075 targets the GLP-1R and is intended to treat endocrinology and metabolic diseases, with a specific focus on obesity and metabolic diseases. It has reached Phase 2 in both global and Chinese clinical trials, indicating that it has shown promise in earlier stages of development and is now being further evaluated for its safety and efficacy.