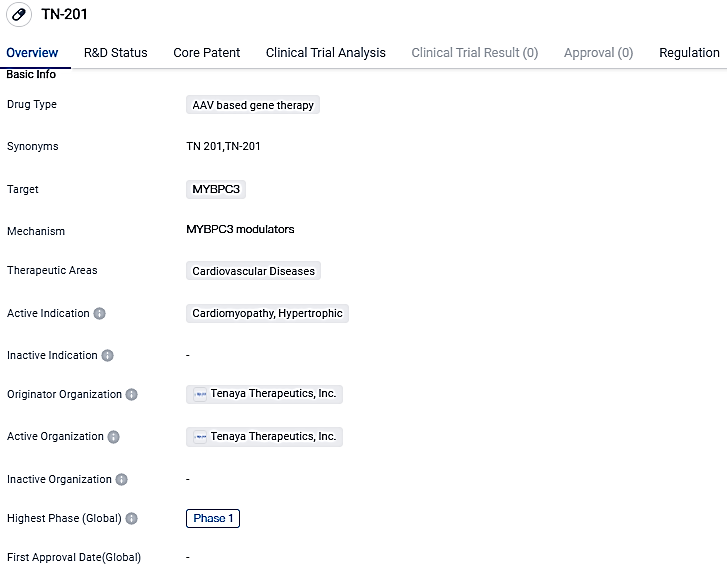
First patient given TN-201 in Tenaya Therapeutics' MyPeak-1™ Phase 1b trial for MYBPC3-related Hypertrophic Cardiomyopathy
Tenaya Therapeutics, Inc., a clinical-stage biotech firm committed to find, create and offer probably curative treatments targeting the root causes of heart disease, has declared the initiation of dosing for the inaugural patient with TN-201 gene therapy. This treatment is designed to help individuals suffering from Myosin Binding Protein C3 (MYBPC3)-linked HCM and it's being carried out in the MyPeak-1 Phase 1b clinical study at the Cleveland Clinic located in Cleveland, Ohio. Tenaya foresees the release of preliminary data from the MyPeak-1 experiment in 2024.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TN-201 is a gene therapy under development by Tenaya that leverages the use of an adeno-associated virus to transport a functioning copy of the MYBPC3 gene directly into heart muscle cells. The goal of this process is to re-establish normal myosin-binding protein levels that play an integral role in the contraction and relaxation of heart muscle cells. Preliminary research has shown that TN-201 not only prevents the progression of the disease, but also significantly reverses it, increasing survival rates after a single dose.
"Mutations in the MYBPC3 gene are the predominant cause of HCM genetically, and individuals with HCM related to MYBPC3 face an elevated risk of rapid deterioration and associated severe complications," stated Milind Desai, M.D., MBA, Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic and Vice Chair.
Dr. Milind Desai added, “TN-201, being a gene therapy for treating MYBPC3-related HCM, holds out the promise of a singular treatment to rectify the genetic basis of the disease, thus enhancing patient results. We are delighted to be a part of the inaugural human clinical trial of TN-201, as another viable application for gene therapy is explored.”
The MyPeak-1 Phase 1b clinical study is a multi-institutional, non-randomized, dose-increasing trial, designed to evaluate the safety, bearability, and therapeutic success of a one-shot IV infusion of TN-201. Initially, this trial aims to involve at least six people who display signs of illness, have been ID'd with MYBPC3-associated nonobstructive HCM and possess an implanted cardioverter defibrillator, expanding potentially to treat a total of 15 subjects both within the U.S. and globally.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
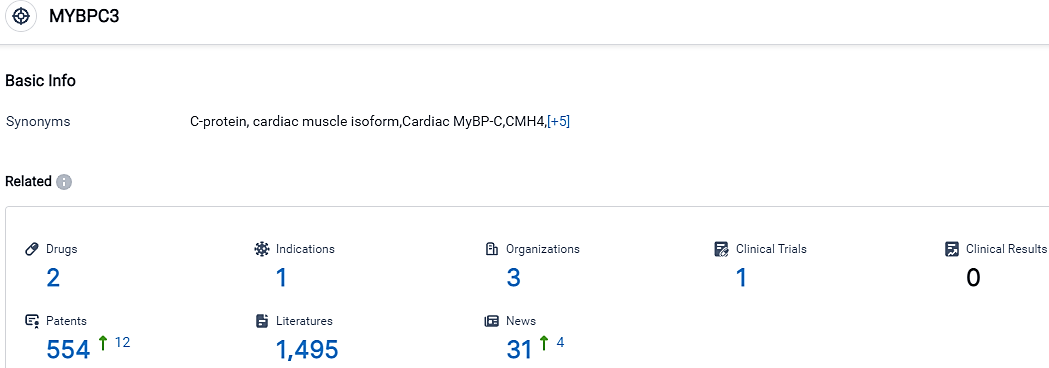
According to the data provided by the Synapse Database, As of October 17, 2023, there are 2 investigational drugs for the MYBPC3 target, including 1 indications, 3 R&D institutions involved, with related clinical trials reaching 1,and as many as 554 patents.
The experimental AAV-based gene therapy, TN-201, is a first-of-its-kind treatment under development for HCM, caused by MYBPC3 gene variations. It has received Fast Track and Orphan Drug status from the FDA and has been recognized as an orphan medicinal product by the European Commission.