Get the full information on benegrastim
Benegrastim, also known as F-627, is a fusion protein targeting CSF-3R (GCSFR). Developed by Evive Biotech.Benegrastim is used for the treatment of neutropenia, fever, breast cancer, Parkinson's disease, and other conditions. On May 6, 2023, it received regulatory approval in China for the prevention and treatment of chemotherapy-induced neutropenia in cancer patients.
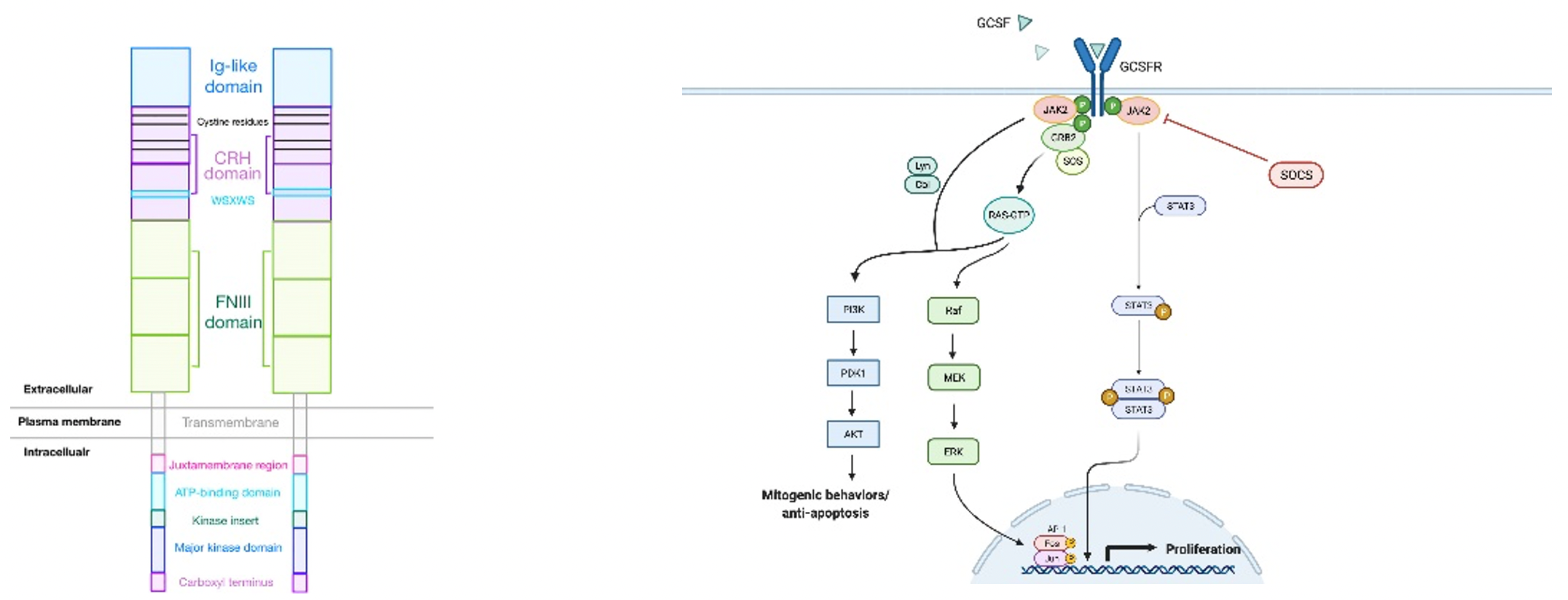
Granulocyte colony-stimulating factor (G-CSF/CSF3) is a multifunctional cytokine produced by the transcription of the CSF3 gene. G-CSF, a hematopoietic growth factor, binds to its receptor, CSF-3R, encoded by CSF3R, to regulate the vitality, proliferation, and differentiation of granulocyte precursors, as well as the function of neutrophils.
CSF-3R is expressed on subsets of neutrophils, monocytes/macrophages, hematopoietic stem/progenitor cells (HSPCs), B lymphocytes, and NK cells. However, recent studies have found that abnormal expression and signaling of CSF-3R may have potential oncogenic effects in several hematological malignancies and solid tumors.
Specifically, overexpression of CSF-3R has been observed in cancer cells of nasopharyngeal carcinoma, oral cancer, breast cancer, colorectal cancer, and ovarian cancer, suggesting a potential role of CSF-3R in cancer progression [1,2,3,4].
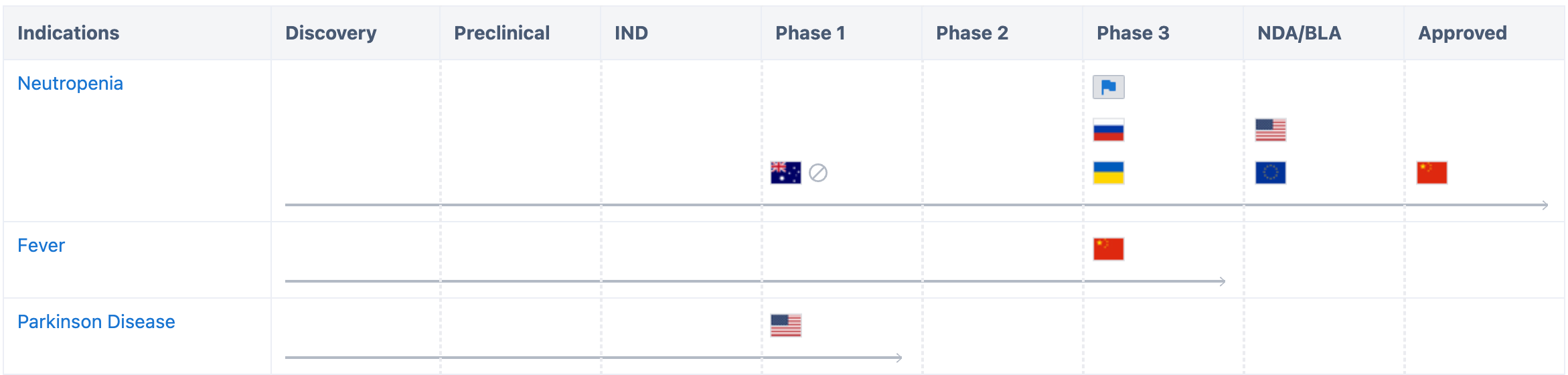
According to data from Synapse, regarding the development of neutropenia, aside from being approved in China, it is undergoing market application in the United States and the European Union. It has entered Phase III clinical trials in five countries including Russia and Ukraine. Fever-related trials have entered Phase III clinical trials in China.
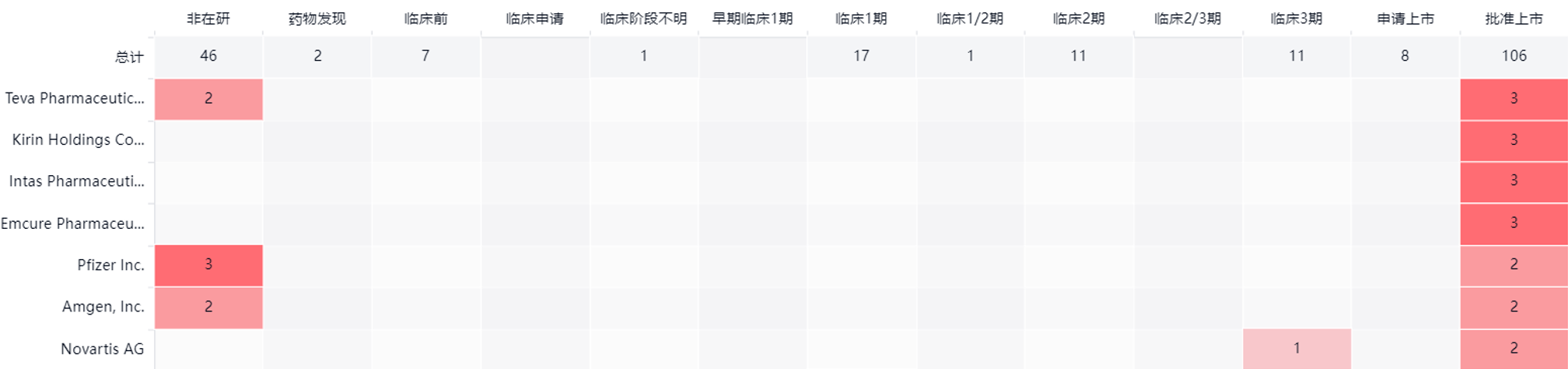
According to data from Synapse, Benegrastim is involved in a total of 15 indications and has all entered the clinical trial stage.

The data submitted for market application is mainly based on the trial data from NCT03252431 and NCT02872103. These two trials respectively evaluated the efficacy and safety of Benegrastim in adult women with stage I-III and stage II-IV breast cancer receiving chemotherapy.
In the NCT03252431 study, patients were randomly assigned to the Benegrastim group or the Neulasta group. In the NCT02872103 study, patients were randomly assigned to receive Benegrastim or placebo. The primary endpoint of these two studies was the duration of Grade 4 neutropenia during the first chemotherapy cycle, defined as the number of days with an absolute neutrophil count below 0.5×109/L.
The results showed that the average duration of Grade 4 neutropenia in both the Benegrastim group and the Neulasta group was 0.2 days, while compared to 3.9 days in the placebo group, the average duration of Grade 4 neutropenia in patients receiving Benegrastim treatment was 1.3 days (P<.0001).
The NCT03252431 study showed that among the subjects who experienced all-cause mortality, there was 1 out of 197 subjects (0.51%) in the Benegrastim group and 2 out of 196 subjects (1.02%) in the Neulasta group. The incidence of serious adverse events (SAEs) was 12/197 (6.09%) in the Benegrastim group and 3/196 (1.53%) in the Neulasta group.
The NCT02872103 study showed that there were no subjects who experienced all-cause mortality in either the Benegrastim or placebo group during cycles 1-4 of chemotherapy. For the four cycles of chemotherapy, the incidence of SAEs was 4/83 (4.82%) in the Benegrastim group and 11/39 (28.21%) in the placebo group.
According to a search in Synapse, the authorized patent related to the product is titled "Recombinant Human G-CSF Dimer and Its Use in the Treatment of Neurological Disorders." The invention provides a recombinant human granulocyte colony-stimulating factor (rhG-CSF) dimer with an extended serum half-life and enhanced biological activity compared to G-CSF.
The dimer is formed by pairing two G-CSF-Fc complexes through disulfide bonds of the Fc fragment. The G-CSF-Fc complex includes a G-CSF monomer, an Fc fragment of IgG2, and a peptide linking the G-CSF monomer and Fc fragment. The rhG-CSF can be used for the treatment of neurological disorders. The patent number is CN102906120A (valid), and the application date is May 25, 2011.
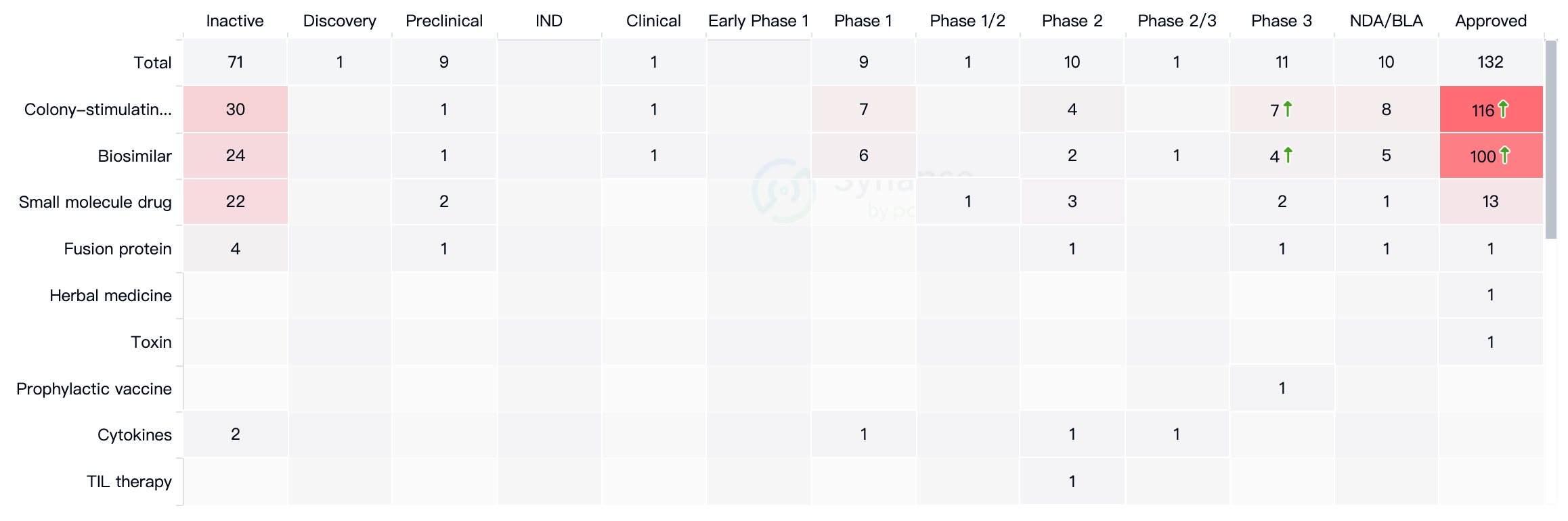
According to data from Synapse, as of May 12, 2023, there are over a hundred drugs approved for marketing worldwide that are related to neutropenia, with the majority being biosimilars. The main type of drug is colony-stimulating factors, with a total of 116 drugs in this category. The drug target is primarily CSF-3R, with 103 drugs already on the market, followed by CSF-2R, with 14 drugs currently on the market.

References
1.Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES, et al. G-CSF promotes neuroblastoma tumorigenicity and metastasis via STAT3-dependent cancer stem cell activation. Cancer Res (2015) 75:2566–79. doi: 10.1158/0008-5472.CAN-14-2946
2.Wang L, Arcasoy MO, Watowich SS, Forget BG. Cytokine signals through STAT3 promote expression of granulocyte secondary granule proteins in 32D cells. Exp Hematol (2005) 33:308–17. doi: 10.1016/j.exphem.2004.11.014
3.Wojtukiewicz MZ, Sierko E, Skalij P, Kaminska M, Zimnoch L, Brekken RA, et al. Granulocyte-colony stimulating factor receptor, tissue factor, and VEGF-r bound VEGF in human breast cancer in loco. Adv Clin Exp Med (2016) 25:505–11. doi: 10.17219/acem/62398
4.Touw IP, Van De Geijn GJ. Granulocyte colony-stimulating factor and its receptor in normal myeloid cell development, leukemia and related blood cell disorders. Front Biosci (2007) 12:800–15. doi: 10.2741/2103
5.https://clinicaltrials.gov/ct2/show/results/NCT03252431?term=NCT03252431&draw=2&rank=1
6.https://clinicaltrials.gov/ct2/show/results/NCT02872103?term=NCT02872103&draw=2&rank=1&view=results