GIBF Invests $10 Million in Nectin Therapeutics for Novel Immunotherapies and ADCs
The Guangzhou-Israel Biotechnology Fund has confirmed its investment of $10 million into Nectin Therapeutics Ltd. Nectin specializes in the creation of innovative targeted immunotherapies, which combat resistance to existing immune oncology therapies.
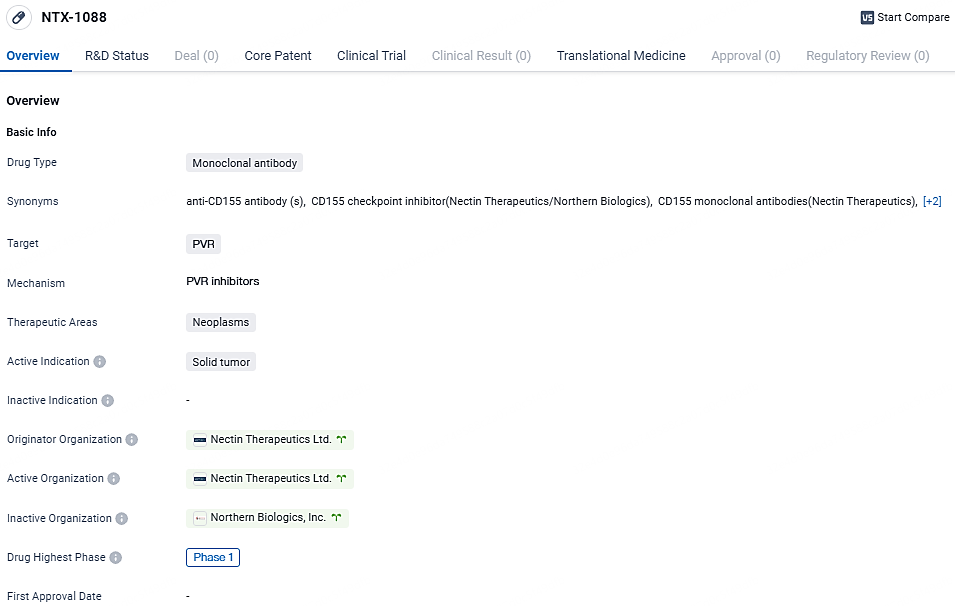
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The allocated resources are set to advance the ongoing projects under Nectin’s innovative immuno-oncology range, notably propelling the global Phase 1 clinical trial of NTX1088, which targets PVR, and the preclinical progress of its anti-drug conjugate collection.
Nectin, a clinical-stage biotech enterprise, is committed to revolutionizing cancer care by exploiting novel insights into nectin pathways to craft cutting-edge immune oncology treatments. The company's unique therapies are poised to establish groundbreaking standards in treatment effectiveness and patient success rates for a variety of challenging cancer types.
Nectin's technologies combat the key resistance mechanisms found in existing IO therapies with an expansive portfolio comprising new monoclonal antibodies and antibody-drug conjugates. With a prestigious team of experts in scientific research and management, deeply rooted in oncology drug development, Nectin holds a track record of excellence in nurturing biotech ventures and crafting groundbreaking treatments. This venture-supported, privately-owned entity garners financial backing from notable investors like aMoon Fund, Peregrine Ventures, IBF, Integra Holdings, Myeloma Investment Fund, and Cancer Focus Fund.
NTX1088, a pioneering monoclonal antibody developed by Nectin, specifically targets PVR—a membrane protein on cancer cells linked to resistance against PD1 and PDL1 immune checkpoint inhibitors. This novel approach of blocking PVR by NTX1088 strives to revive antitumor immune activity via DNAM1—the first of its kind in therapeutic strategies.
DNAM1, a critical glycoprotein on the surface of T and NK cells, is inhibited by PVR in tumor cells. Interfering with PVR, thereby enhancing DNAM1's expression and functionality, amplifies the antitumor impact of T and NK cells. Further, NTX1088’s blockade of PVR boosts the immune response against tumors by halting the inhibitive signals from several immune checkpoints, including TIGIT and CD96.
Professor Shlomo Noy, Managing Partner and Chief Medical Officer at GIBF, expressed his enthusiasm for Nectin's pioneering work. "Nectin is at the forefront of developing breakthrough immune-based cancer treatments. We are thrilled to support their vital goals and team. The strides this company is making in its research and development are leading to transformative treatments that will significantly enhance the treatment experiences and outcomes for numerous cancer patients," he stated.
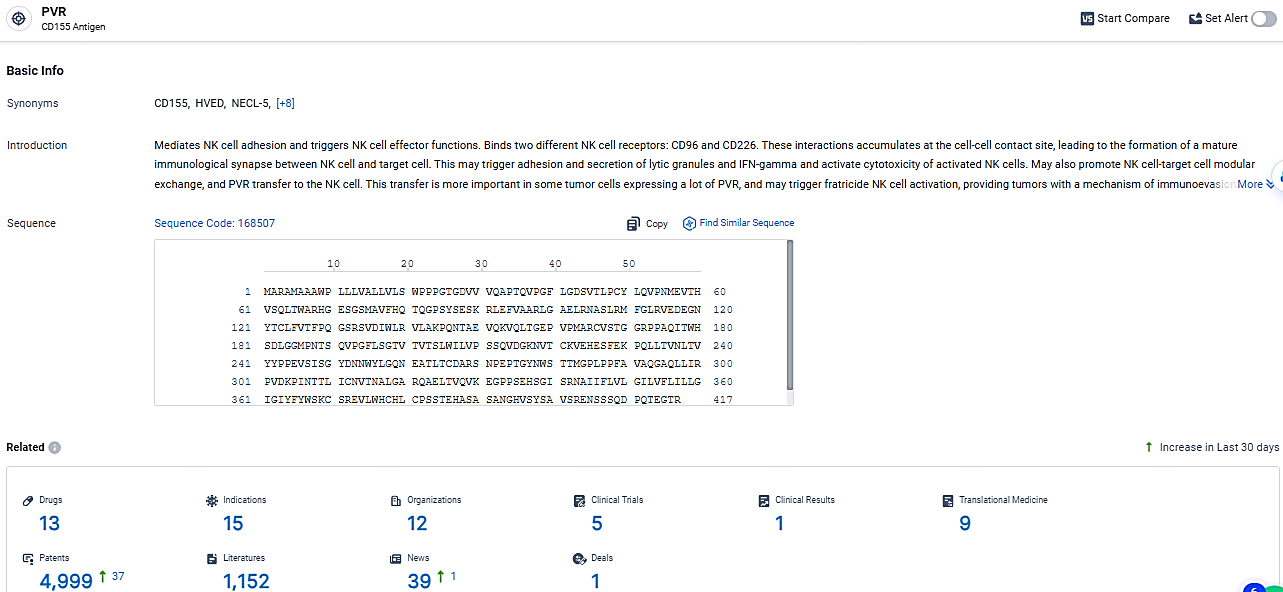
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 7, 2024, there are 13 investigational drugs for the PVR target, including 15 indications, 12 R&D institutions involved, with related clinical trials reaching 5, and as many as 4999 patents.
NTX-1088 targets PVR and is intended for the treatment of solid tumors. Currently in Phase 1 of clinical development, NTX-1088 shows promise in potentially inhibiting tumor growth and improving patient outcomes in the field of neoplasms.