Edgewise Therapeutics Starts Phase 2 Trial of EDG-7500 for Obstructive HCM
Edgewise Therapeutics, Inc., a prominent biopharmaceutical enterprise specializing in muscle disorders, revealed the initiation of the first patient dosing in the Phase 2 CIRRUS-HCM trial for their drug EDG-7500. EDG-7500, an innovative oral compound, acts as a selective modulator of the cardiac sarcomere. It is tailored to decrease the early velocity of contraction and alleviate the issues related to cardiac relaxation seen in HCM and additional diastolic dysfunction conditions.
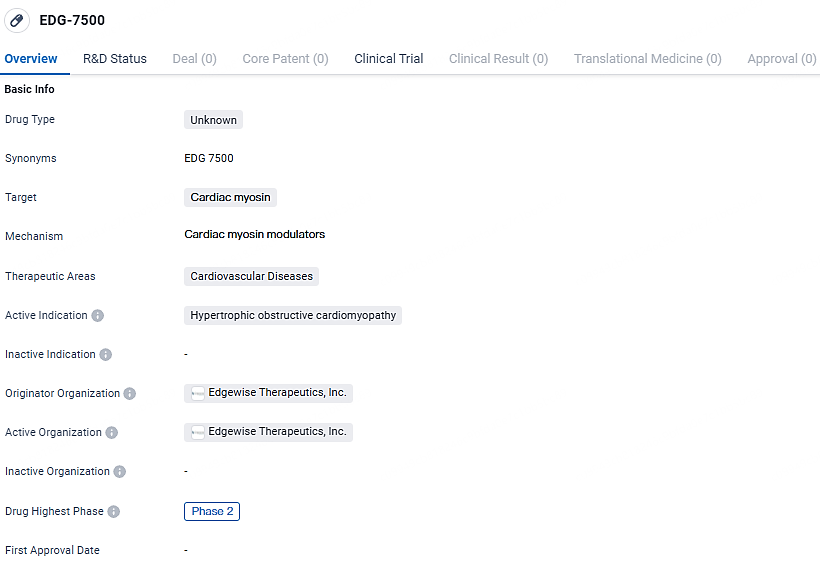
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase 2 study aims to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of EDG-7500 in individuals diagnosed with obstructive HCM. The study will be conducted in two parts; Part A will focus on the effects of single dose administrations, whereas Part B will explore the consequences of multiple oral doses administered over a span of 28 days.
Marc Semigran, M.D., Chief Development Officer at Edgewise Therapeutics, expressed optimism regarding preliminary findings, stating, "In a relevant animal model of obstructive HCM, EDG-7500 effectively reduced the gradient at the left ventricular outflow tract and maintained normal LV contractility levels. Notably, EDG-7500 administration, in both short-term and long-term durations, in models representing non-obstructive HCM, displayed substantial enhancements in both ventricular filling and diastolic function."
Further, Kevin Koch, Ph.D., President and CEO of Edgewise Therapeutics, emphasized the significance of progressing EDG-7500 studies in patients with obstructive HCM: "This step is a critical achievement for Edgewise and reflects our solid expertise in discovery and development, underscoring our dedication to addressing severe muscular disorders."
Edgewise Therapeutics anticipates releasing findings from the single dose segment of this trial alongside data from a Phase 1 study involving healthy volunteers during the third quarter of 2024. Additionally, there are plans to commence a 28-day study including patients with both obstructive and non-obstructive HCM in the latter half of 2024. An open-label extension study for EDG-7500 is also projected to begin in the last quarter of 2024.
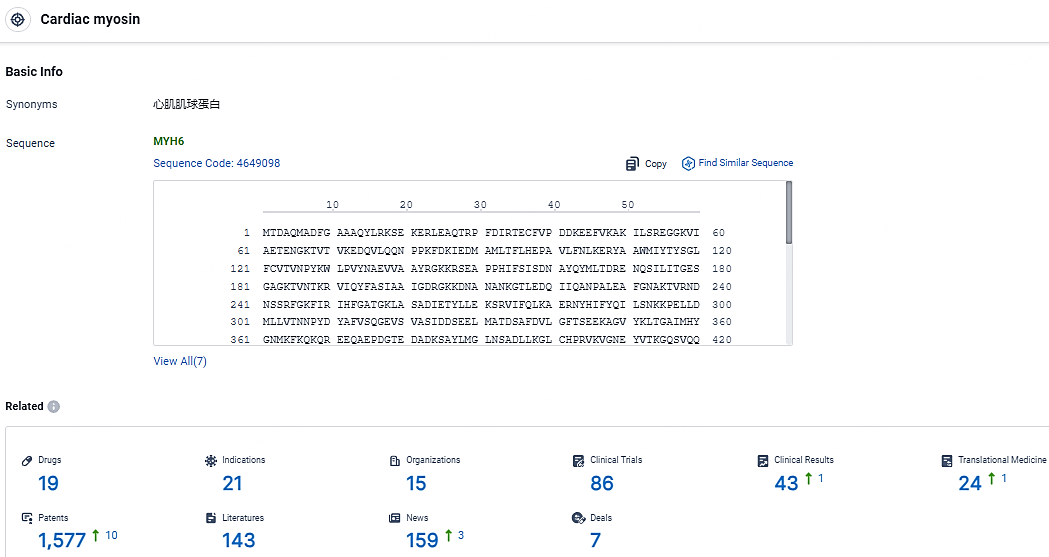
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 6, 2024, there are 19 investigational drugs for the cardiac myosin targets, including 21 indications, 15 R&D institutions involved, with related clinical trials reaching 86, and as many as 1577 patents.
EDG-7500 is a drug developed by Edgewise Therapeutics, Inc. that targets cardiac myosin and aims to address hypertrophic obstructive cardiomyopathy. With its current status in Phase 2 of development, EDG-7500 shows promise as a potential treatment option for patients suffering from this cardiovascular disease. Further research and clinical trials will be necessary to determine the drug's safety and efficacy before it can be made available to patients.