Fate Therapeutics Presents Preliminary FT522 Data for Autoimmune Diseases at ASGCT
Fate Therapeutics, Inc., a company engaged in clinical-stage biopharmaceutical development, focusing on novel induced pluripotent stem cell (iPSC)-based cellular immunotherapies for cancer and autoimmune disorders, disclosed that an abstract containing groundbreaking preclinical outcomes from its FT522 program targeting autoimmune diseases will be presented at the upcoming 27th Annual Meeting of the American Society of Gene and Cell Therapy. This significant event is scheduled to take place in Baltimore, Maryland from May 7 to May 11, 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
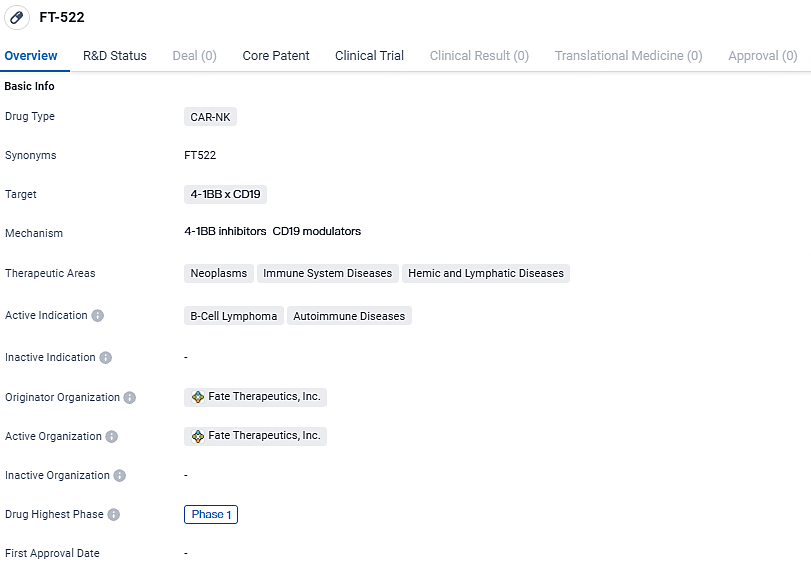
FT522, developed by the Company, is an innovative CAR NK cell therapy product aimed at CD19. It is derived from iPSC technology and incorporates advanced alloimmune defense receptor technology. This technology is intended to enhance the effectiveness of readily available cell therapies, allowing for the treatment of patients without the need for conditioning chemotherapy.
During the ASGCT event, the Company plans to showcase various preclinical studieshighlighting the effectiveness of FT522 applied to peripheral blood mononuclear cells from non-matched donors who are affected by systemic lupus erythematosus. The findings from these studies suggest significant reductions in B-cell populations, prolonged functional persistence, and the suppression of hostile immune reactions. This highlights FT522's potential in effectively managing autoimmune conditions even in the absence of conditioning chemotherapy.
The unique potential of human induced pluripotent stem cells includes endless self-renewal and the capability to differentiate into any cell type present in the human body. The Company has developed a specialized iPSC platform that employs sophisticated multiplexed-engineering of human iPSCs alongside single-cell selection to produce standardized master iPSC lines. These lines serve as foundational cell sources similar to the master cell lines employed for the large-scale production of biopharmaceuticals, such as monoclonal antibodies. Utilizing these master iPSC lines, the Company can produce uniform and precisely defined engineered cells. These cells are readily storable for instant utilisation, compatible with other therapeutic procedures, and have the potential to be used across a diverse range of patients.
Hence, this platform offers a solution to several challenges in producing cell therapies from cells obtained from patients or donors. Supported by over 500 granted patents and another 500 pending applications, the intellectual property of Fate Therapeutics’ iPSC platform secures its innovative approach to cell therapy manufacturing.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
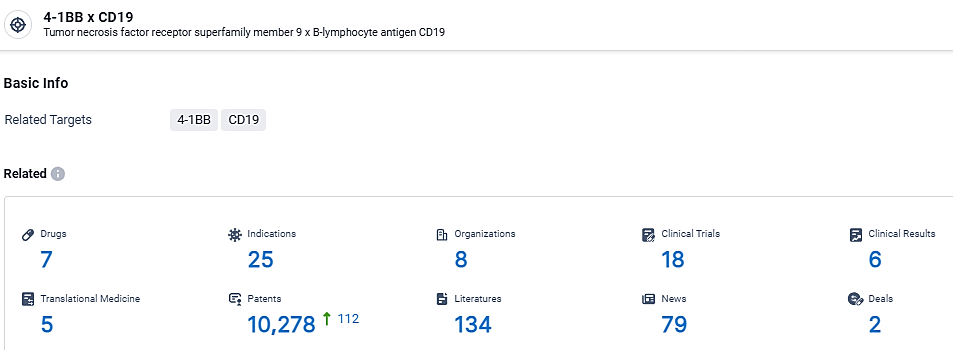
According to the data provided by the Synapse Database, As of May 6, 2024, there are 7 investigational drugs for the 4-1BB and CD19 target, including 25 indications, 8 R&D institutions involved, with related clinical trials reaching 18, and as many as 10278 patents.
FT-522 targets the proteins 4-1BB and CD19 and has potential applications in treating neoplasms, immune system diseases, and hemic and lymphatic diseases. The active indications for FT-522 are B-cell lymphoma and autoimmune diseases. Currently, FT-522 is in Phase 1 of clinical development.