GigaGen Receives Authorization from FDA to Initiate Phase 1 Study of Cancer Drug GIGA-564 for Solid Tumor Treatment
GigaGen Inc., an innovative enterprise specializing in the development of groundbreaking antibody therapeutics targeting immune disorders, contagions, and tumors unresponsive to checkpoint inhibitors, and an affiliate of the healthcare firm Grifols, has disclosed receiving authorization from the U.S. Food and Drug Administration for its Investigational New Drug submission. This clearance paves the way for the commencement of a Phase 1 clinical study, which will assess the efficacy of GigaGen’s cancer drug candidate, GIGA-564, in the management of various solid malignancies.
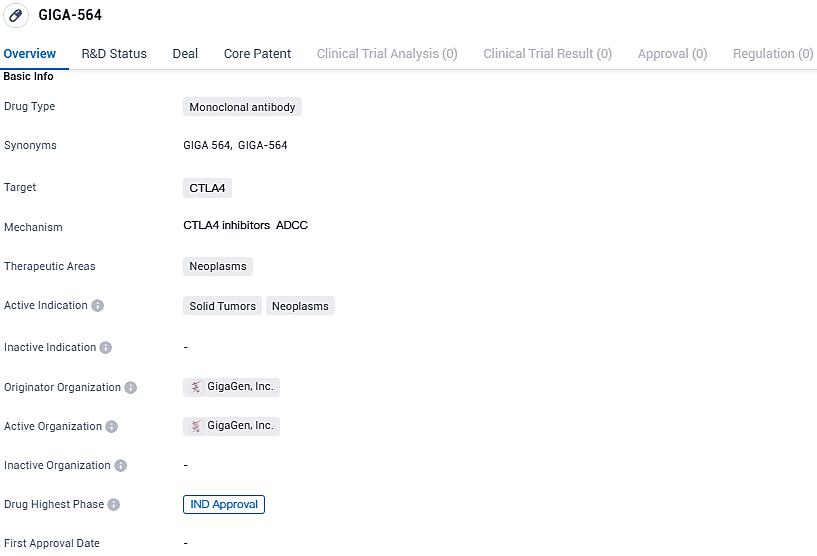
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"It brings us great satisfaction to announce we've achieved this critical juncture, which will allow our premier oncology product, GIGA-564, to proceed to clinical trials," announced Carter Keller, Grifols' senior vice president and the leader of GigaGen. "GIGA-564 embodies an innovative strategy for CTLA-4 engagement, potentially offering heightened anti-cancer effects and a reduction in the adverse side effects observed with current anti-CTLA-4 therapies. We're eager to commence the trial in 2024 and are hopeful that this advancement will materialize into real-world health benefits for those affected by cancer."
To date, preclinical studies have shown that GIGA-564 can deplete T regulatory cells present within the tumor milieu, augmenting the destructive actions of effector T cells against cancer cells. This has led to a leap in cancer-fighting efficiency and a decrease in harmful side effects when contrasted with the established CTLA-4 inhibitory monoclonal antibody, ipilimumab.
The early-phase clinical trial, staged as 1a/1b, is set to assess the safety and efficacy of GIGA-564 in treating patients with advanced solid tumors. This research will be spearheaded by scientists from the National Cancer Institute of the National Institutes of Health, collaborating closely with the GigaGen research team.
Pioneering a distinct pathway in anti-CTLA-4 therapy, GIGA-564 is a fully human monoclonal antibody with potential advantages. In contrast, previous anti-CTLA-4 therapeutics relied on the potent interruption of CTLA-4's binding to its ligands, thus promoting T cell activation.
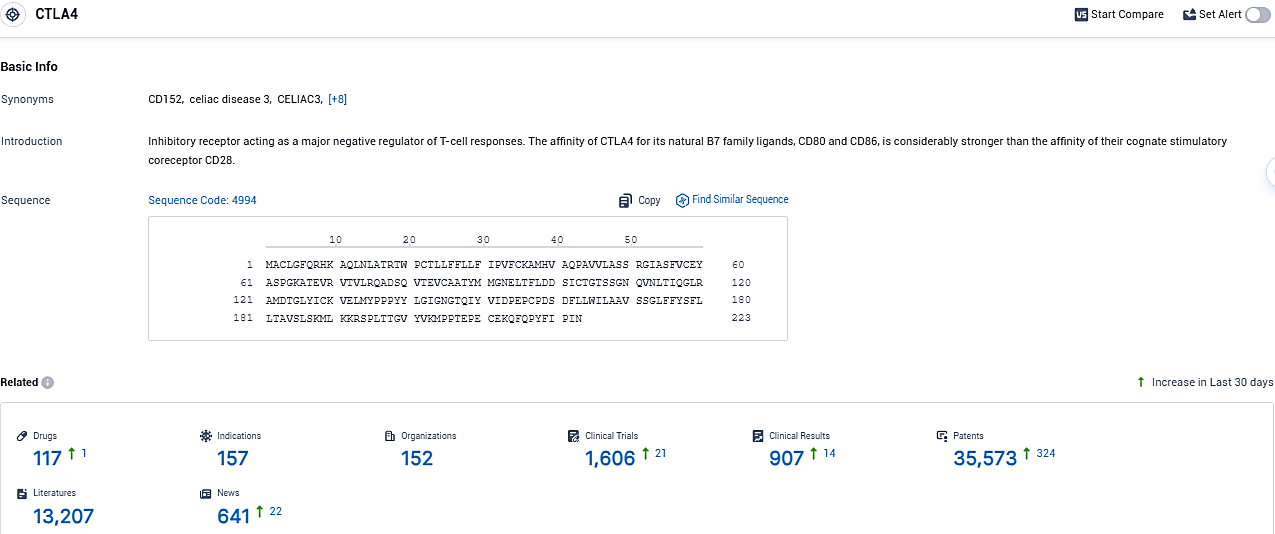
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 117 investigational drugs for the CTLA4 target, including 157 indications, 152 R&D institutions involved, with related clinical trials reaching 907, and as many as 35573 patents.
GIGA-564 targets CTLA4 and is indicated for the treatment of solid tumors and neoplasms. With its focus on neoplasms and solid tumors, GIGA-564 shows promise as a potential treatment for various types of cancer. The drug has reached the IND approval phase, indicating its readiness for clinical trials.