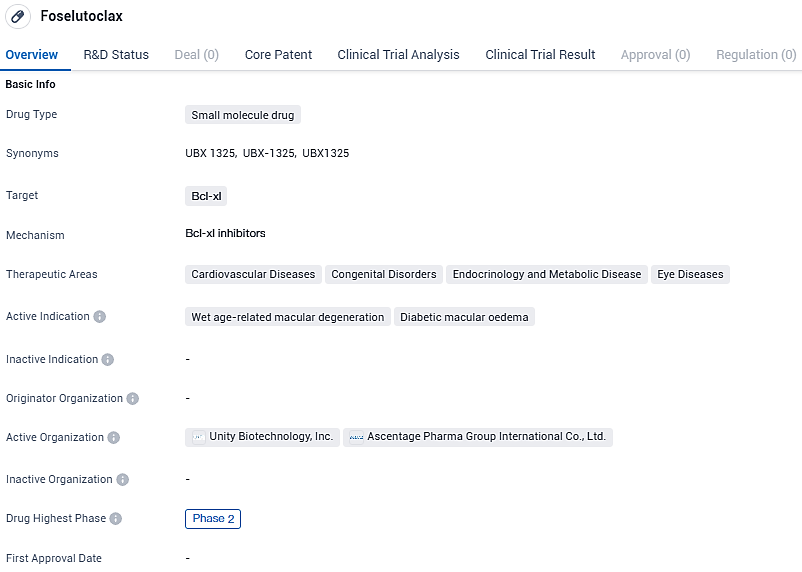
UNITY Biotechnology Begins Phase 2 ASPIRE Trial Administering UBX1325 to Initial Participants with Diabetic Macular Edema
Biopharmaceutical enterprise UNITY Biotechnology, Inc., which specializes in creating treatments designed to decelerate, stop, or revert age-related conditions, has disclosed the initiation of treatment for the initial participants in their Phase 2 ASPIRE trial. This study is investigating the efficacy of UBX1325 (foselutoclax), a therapeutic agent targeting Bcl-xL, and it is being directly compared to conventional anti-VEGF therapies in individuals affected by diabetic macular edema.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"In spite of receiving regular anti-VEGF treatments, numerous DME sufferers continue to experience suboptimal vision, highlighting the critical demand for alternative therapeutic strategies," stated UNITY's CEO, Anirvan Ghosh, Ph.D. "The medical community has shown considerable enthusiasm for the healing possibilities presented by UBX1325, which is founded on an innovative senolytic mode of action. We are eager to unveil preliminary findings from the ongoing research by the end of 2024."
ASPIRE is a multicentric, placebo-controlled, randomized trial with double blinding, actively comparing the safety and therapeutic impact of UBX1325 against aflibercept in patients with active diabetic macular edema who have not responded adequately to standard treatments. It aims to recruit approximately 40 participants who will undergo random allocation at a ratio of 1:1 to receive either 10 μg UBX1325 or the 2 mg aflibercept standard treatment through injections at intervals of eight weeks for a duration of six months.
The main measure of efficacy for the trial is the average change in Best Corrected Visual Acuity from the initial measurement to week 24. Other measures of interest include the variance in BCVA over the study period, as well as alterations in the central subfield thickness from the starting point to week 24. Interim data at 16 weeks is anticipated to be released toward the end of 2024, with further data at 24 weeks slated for publication in the early months of 2025.
Currently under investigation, UBX1325 is a candidate pharmaceutical targeting retinal disorders such as DME and wet AMD, but has not received approval for use anywhere globally. It functions as a highly potent inhibitor of the protein Bcl-xL, which belongs to the Bcl-2 family known for their role in regulating apoptosis.
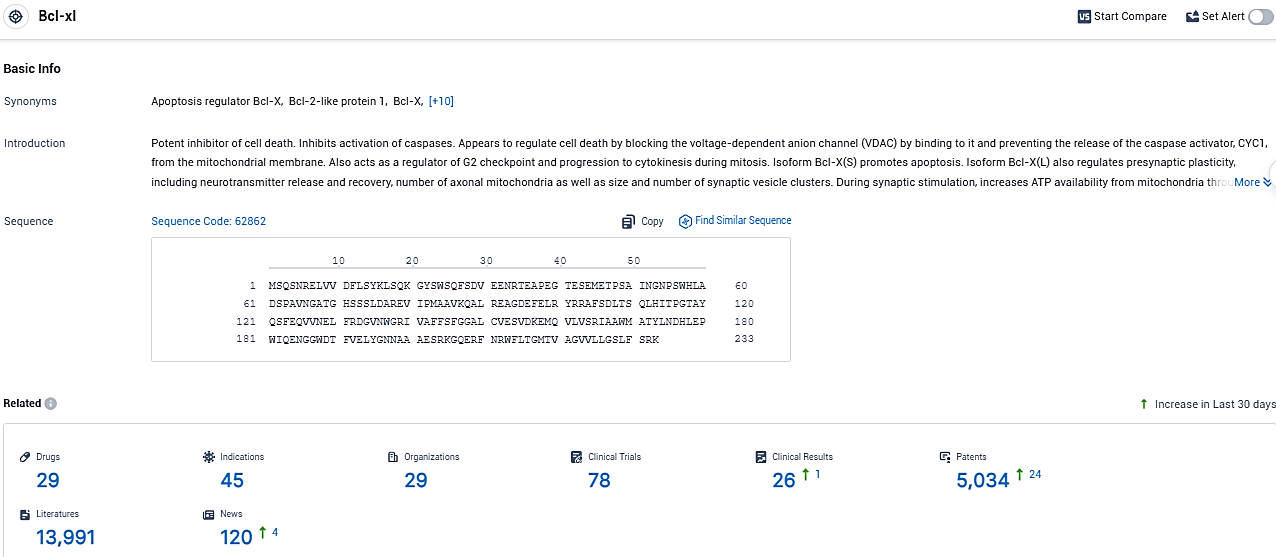
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 29 investigational drugs for the Bcl-xl target, including 45 indications, 29 R&D institutions involved, with related clinical trials reaching 78, and as many as 5034 patents.
UBX1325 is designed to inhibit the function of proteins that senescent cells rely on for survival. In preclinical studies, UNITY has demonstrated that targeting Bcl-xL with UBX1325 preferentially eliminated senescent cells from diseased tissue while sparing cells in healthy tissue. UNITY’s goal with UBX1325 is to transformationally improve real-world outcomes for patients with retinal disease.