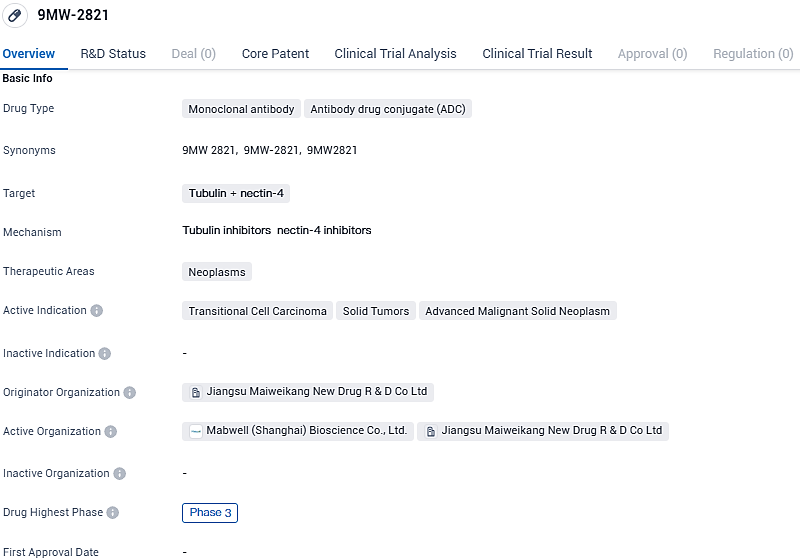
Mabwell Reveals Regulatory Green Light for Pioneering Nectin-4 Specific Antibody-Drug Conjugate Entering Stage 3 Research Trials
Mabwell has disclosed that its application for conducting the Phase III clinical trial, titled "A Randomized, Open-label, Controlled, Multicenter Study Comparing 9MW2821 to Investigator-Selected Chemotherapy in the Treatment of Inoperable Advanced Locally or Metastatic Urothelial Carcinoma in Patients Who Have Prior Treatment with Platinum-Based Chemotherapy and PD-(L)1 Inhibitors," has received the green light from the Center for Drug Evaluation of the National Medical Products Administration.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mabwell has embarked on the commencement of a critical Phase III clinical trial for its innovative drug 9MW2821. This trial targets the treatment of urothelial carcinoma that is either locally advanced or metastatic and has been observed in individuals who have undergone prior treatment involving platinum-based chemotherapy as well as PD-(L)1 inhibitor regimens.
This particular drug, 9MW2821, represents a groundbreaking Antibody-Drug Conjugate (ADC) with Nectin-4 as its designated target, distinguishing itself as the inaugural ADC within China to focus on such a target and advance into clinical evaluation. By the date of December 5, 2023, the trial had successfully enlisted in excess of 250 participants.
During the Phase II clinical evaluation for 9MW2821, when administered at a dosage of 1.25 mg/kg, impressive outcomes were revealed with the standalone usage of the drug. Collected data presented an objective response rate of 62.2% and a disease control rate of 91.9% among subjects with advanced stage urothelial carcinoma. Moreover, the median duration of progression-free survival recorded was 6.7 months, and the average overall survival period had yet to be determined.
Presently, this promising drug is at the center of a series of clinical trials that are progressing in tandem. The scope of research is not limited to its effectiveness as a monotherapy but extends to its potential in combination therapies with various other medicinal treatments. Thus far, said drug has exhibited encouraging results in combating tumors and maintaining a satisfactory safety profile across a spectrum of cancer categories.
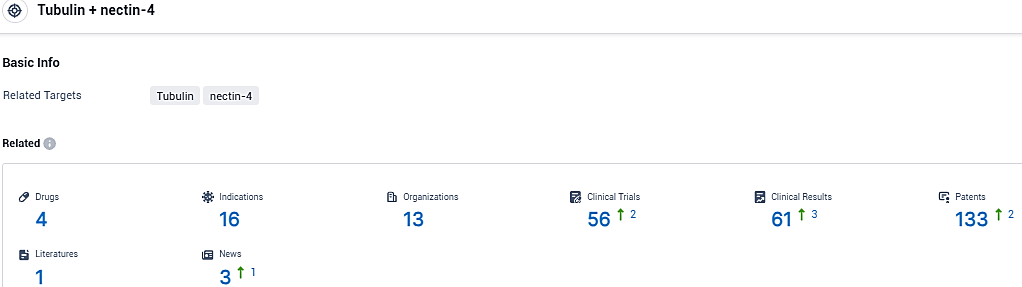
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 17, 2023, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 16 indications, 13 R&D institutions involved, with related clinical trials reaching 56, and as many as 133 patents.
9MW2821 is a novel Nectin-4 targeting ADC developed by world-class ADC development platform and automated high-throughput antibody discovery platform of Mabwell. Currently, the R&D progress of 9MW2821 ranks first in China and second in the world. 9MW2821 is the first to read out preliminary clinical data in cervical cancer among the products with the same target in the world.