Global New Drug Research and Development Progress Weekly Report (10.28-11.3)
What drugs have been approved this week?
1. Novartis: FDA Approves New First-Line Indication for Bcr-Abl Inhibitor
On October 29, Novartis announced that the FDA has granted accelerated approval for a new indication for Scemblix (Asciminib) to treat newly diagnosed adult patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP).
Previously, in October 2021, Scemblix was approved in the U.S. for adult patients with Ph+ CML-CP who have undergone at least two tyrosine kinase inhibitor (TKI) treatments or possess the T315I mutation. The drug later received approval in Japan and the European Union in March and August 2022, respectively. According to the press release, expanding the treatment line is expected to increase the eligible patient population by approximately fourfold.

Scemblix is a STAMP inhibitor developed by Novartis that specifically targets the myristoyl pocket of the BCR-ABL1 protein. The accelerated approval was based on results from the Phase III ASC4FIRST clinical trial, which measured major molecular response (MMR) at week 48. This study compared once-daily Scemblix with standard TKI therapies selected by the investigators (Imatinib, Nilotinib, Dasatinib, or Bosutinib).
Results demonstrated that Scemblix improved MMR rates at week 48 by nearly 20% compared to the standard TKI therapies (68% vs. 49%, p<0.001) and by nearly 30% compared to Imatinib monotherapy (69% vs. 40%, p<0.001).
2. CStone Pharmaceuticals: PD-L1 Inhibitor Approved in the UK
On October 31, CStone Pharmaceuticals announced that the UK’s MHRA approved Sugemalimab, a PD-L1 inhibitor, in combination with platinum-based chemotherapy for first-line treatment of metastatic NSCLC in adults without EGFR-sensitizing mutations or ALK, ROS1, or RET genomic alterations.
Sugemalimab was previously approved by the European Commission in July this year. The latest approval is based on findings from the Phase III GEMSTONE-302 trial, a multicenter, randomized, double-blind clinical study evaluating the efficacy and safety of Sugemalimab combined with platinum-based chemotherapy versus placebo with platinum-based chemotherapy in stage IV NSCLC patients. The primary endpoint was progression-free survival (PFS) assessed by investigators.
3. Eli Lilly: Non-Covalent BTK Inhibitor Approved in China
On October 29, China’s NMPA announced the approval of Eli Lilly’s Pirtobrutinib tablets for adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have previously received a Bruton’s tyrosine kinase (BTK) inhibitor.
Pirtobrutinib is a highly selective, reversible oral BTK inhibitor that interacts with water molecules and BTK residues near the ATP binding site, rather than binding to the C481 site, demonstrating potential to overcome resistance to covalent BTK inhibitors. It inhibits both wild-type and C481-mutant BTK.
Originally developed by Redx Pharma, Pirtobrutinib was acquired by Loxo Oncology in July 2017. In January 2019, Eli Lilly acquired Loxo Oncology for $8 billion, securing rights to the drug. In March 2022, Innovent Biologics obtained a priority negotiation right to commercialize Pirtobrutinib in China with an initial payment of $45 million.
4. Hengrui Pharmaceutical: New Indication Approved for Vunakizumab in Ankylosing Spondylitis
On October 29, Hengrui’s Vunakizumab injection was approved in China for a new indication to treat ankylosing spondylitis. Previously, on August 27, Vunakizumab was approved for adult patients with moderate-to-severe plaque psoriasis suitable for systemic therapy or phototherapy, marking this as the second approved indication.
Vunakizumab is an IL-17A-targeting humanized IgG1 monoclonal antibody developed by Hengrui with 0.8% murine components to increase IL-17A affinity while reducing immunogenicity. Compared to already approved Secukinumab and Ixekizumab, Vunakizumab exhibits higher IL-17A affinity and stronger inhibition of IL-17A/IL-17R interaction.
What major clinical trial results were released this week?
1. Novo Nordisk: Semaglutide Phase III Trial for MASH Successful, Regulatory Submission Planned
On November 1, Novo Nordisk reported promising primary results from Part 1 of its ongoing ESSENCE trial. Findings showed semaglutide led to significant improvement in liver fibrosis and alleviation of metabolic dysfunction-associated steatohepatitis (MASH) symptoms. Novo Nordisk plans to submit regulatory applications in the U.S. and Europe in early 2025 for this indication. Detailed results from the ESSENCE study will be presented at a medical conference in 2024, while Part 2 of the study will continue through 2029.
The ESSENCE study is a 240-week, Phase III double-blind clinical trial evaluating the efficacy of a weekly subcutaneous injection of 2.4 mg semaglutide in adults with advanced liver fibrosis (stage 2 or 3) due to MASH. At week 72, 37% of semaglutide-treated patients achieved liver fibrosis improvement, compared to 22.5% in the placebo group, and 62.9% of patients showed MASH resolution, versus 34.1% in the placebo group.
2. Eli Lilly: Donanemab Modified Dose Successful in Phase 3b Alzheimer’s Disease Trial
On October 29, Eli Lilly shared positive results from its Phase 3b TRAILBLAZER-ALZ 6 study. The study, conducted on early Alzheimer’s disease (AD) patients, met its primary endpoint by significantly reducing ARIA-E symptoms by week 24. TRAILBLAZER-ALZ 6 is a randomized, double-blind, global study that explored the incidence of amyloid-related imaging abnormalities (ARIA-E) and amyloid clearance across four adjusted dosing regimens of donanemab in early AD patients.
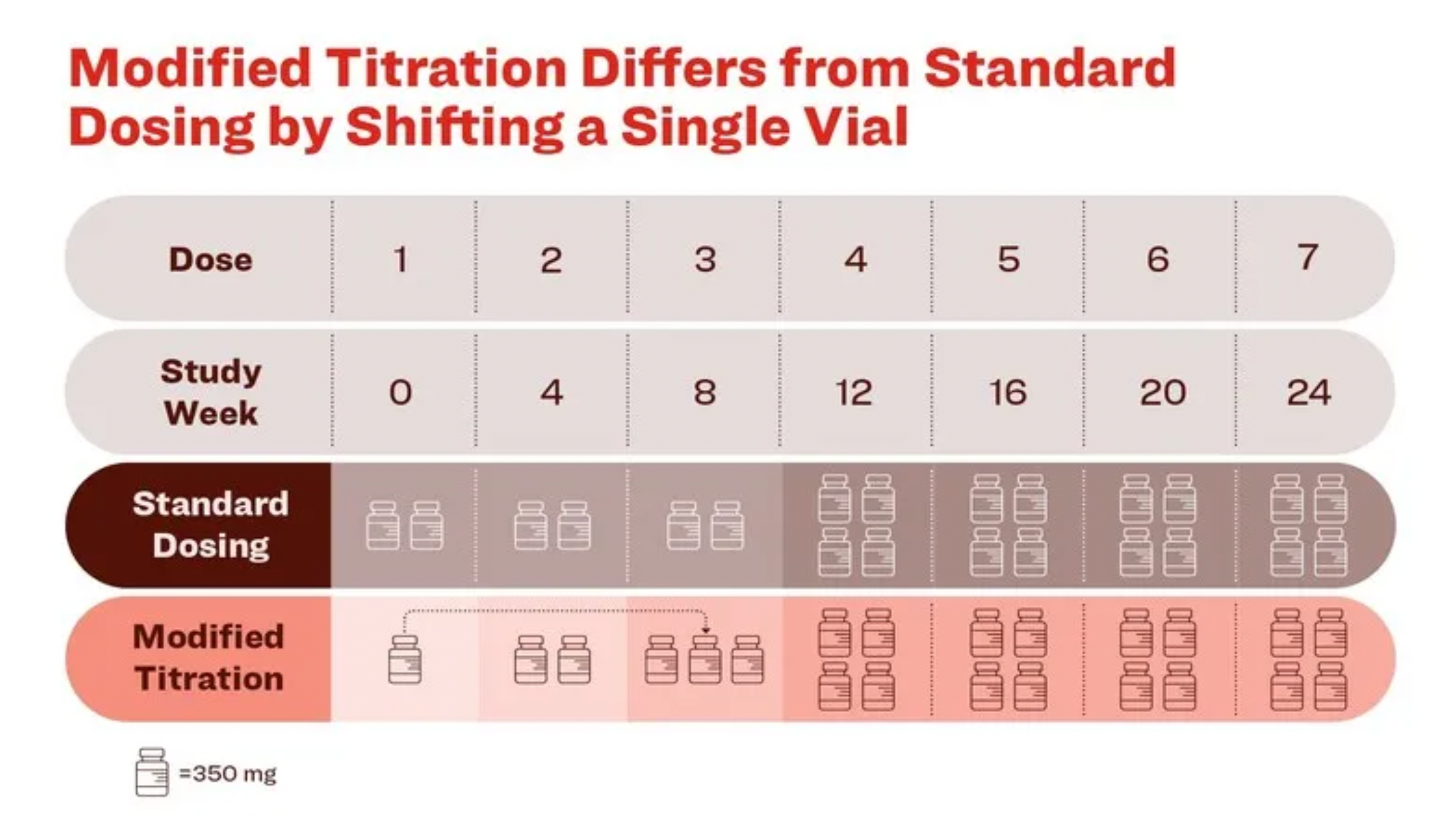
Results showed a 41% reduction in ARIA-E risk with the adjusted regimen (14%) compared to the standard dosing (24%). The adjusted regimen was particularly effective in APOE4 homozygous patients, reducing ARIA-E occurrence by 67% (19% versus 57%).
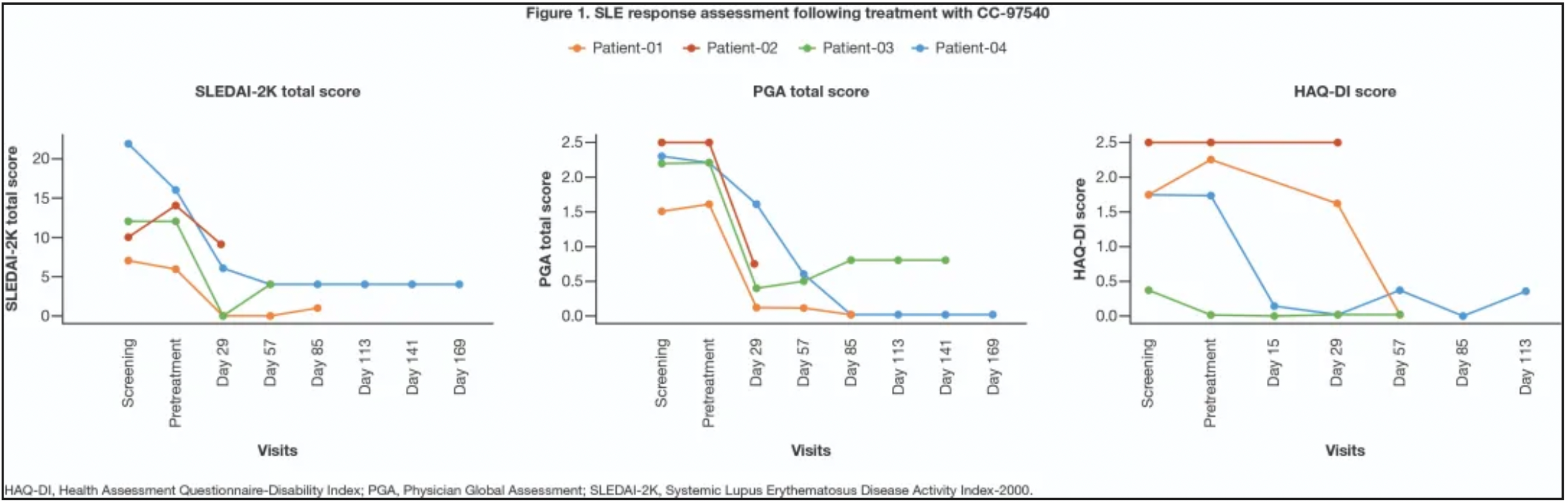
3. BMS Presents CAR-T Therapy Data for Systemic Lupus Erythematosus
Bristol-Myers Squibb will present the initial clinical outcomes of its investigational CD19-targeted CAR-T therapy, CC-97540, at the ACR conference. The study (NCT05869955) included nine patients with severe, refractory autoimmune diseases as of June 2024, with four SLE patients showing kidney involvement that had not responded to previous treatments.
The study reported a mild (Grade 1) cytokine release syndrome in one patient, which resolved within a day without cytokine or corticosteroid intervention. Additionally, three out of four patients (75%) experienced transient lymphopenia, a typical side effect, with no neurotoxicity or serious adverse events observed.
4. Akeso Biopharma: Full Phase III Data for AK111 in Plaque Psoriasis Released
On October 28, Akeso Biopharma announced favorable long-term efficacy and safety results from its AK111-301 Phase III trial of gumokizumab (AK111) for moderate-to-severe plaque psoriasis. Gumokizumab is a humanized IgG1 monoclonal antibody targeting IL-17A, developed to bind to human IL-17A with high specificity and affinity to inhibit psoriasis-associated signaling pathways.
The study followed patients for up to 56 weeks, with findings showing that gumokizumab demonstrated excellent long-term efficacy and a high response rate, providing a promising new treatment option for psoriasis patients.
5. Ji Xing Pharmaceuticals: Successful Phase III Trial of LNZ100 for Presbyopia in China
On October 28, Ji Xing Pharmaceuticals and LENZ Therapeutics announced top-line results from the Phase III JX07001 trial of LNZ100 for presbyopia in China. LNZ100 met its primary and key secondary endpoints, significantly improving near visual acuity (BCDVA) with sustained distance vision for up to 10 hours post-dose in presbyopic patients.
Approximately 84% of participants saw a two-line improvement in near vision, while 69% experienced a three-line improvement within 30 minutes of dosing. At three hours, these improvements were observed in 88% and 74% of patients, respectively, with no severe treatment-related adverse events reported, suggesting LNZ100’s good tolerability.
For more information on the progress of drug development, please consult the Synapse database.