Is Selpercatinib approved by the FDA?
Yes, Selpercatinib, marketed under the brand name Retevmo, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Selpercatinib on May 8, 2020, under an accelerated approval process.
What is Selpercatinib?
Selpercatinib is an oral medication classified as a multikinase inhibitor. It is used to treat specific types of cancer that have spread to other parts of the body (metastatic). The approval is for:
- Non-Small Cell Lung Cancer (NSCLC) in adults
- Thyroid Cancer (including medullary thyroid cancer and RET fusion-positive thyroid cancer) in adults and children aged 12 years and older
How Does Selpercatinib Work?
Selpercatinib targets cancers that have a specific genetic mutation called an abnormal RET gene. This gene fusion drives cancer cell growth, and Selpercatinib inhibits the action of the RET kinase, which is critical for the growth of these cancer cells.
Dosage and Administration
Selpercatinib is available in oral capsule form in two strengths: 40 mg and 80 mg. The dosage depends on the patient’s weight and the type of cancer being treated:
- For patients weighing less than 50 kg: 120 mg orally twice daily
- For patients weighing 50 kg or more: 160 mg orally twice daily
It is important to take Selpercatinib exactly as prescribed by the healthcare provider. The medication is usually taken every 12 hours.
Side Effects
Common side effects of Selpercatinib include:
- Abnormal blood tests
- High blood pressure
- Tiredness
- Dry mouth
- Diarrhea
- Swelling
- Rash
- Constipation
Serious side effects may occur and require immediate medical attention. These include:
- Easy bruising or bleeding
- Coughing up blood or vomit that looks like coffee grounds
- Any wound that will not heal
- Fast or pounding heartbeats
- Liver problems (e.g., jaundice)
Warnings and Precautions
- Selpercatinib can harm an unborn baby. Both men and women should use effective birth control during treatment and for at least one week after the last dose.
- Patients with untreated or uncontrolled high blood pressure should not use Selpercatinib.
- It is essential to inform the healthcare provider of any existing liver disease or a family history of long QT syndrome.
- Breastfeeding is not recommended during treatment and for at least one week after the last dose.
Conclusion
Selpercatinib (Retevmo) is a significant therapeutic option for patients with RET-positive cancers, including non-small cell lung cancer and certain thyroid cancers. Approved by the FDA on May 8, 2020, it offers a targeted approach to treating cancers driven by RET genetic mutations. Patients should follow their healthcare provider’s instructions closely and report any side effects promptly.
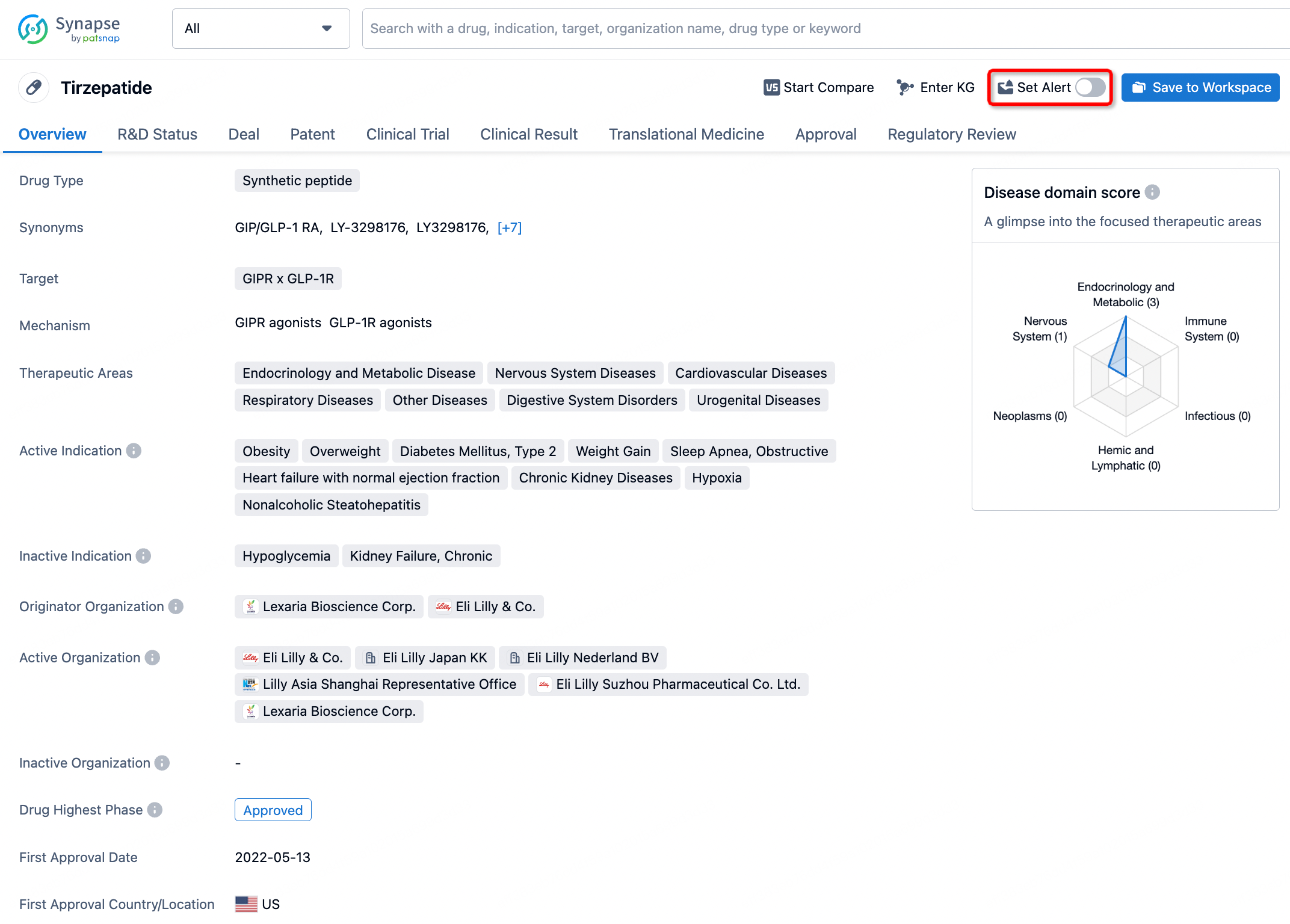
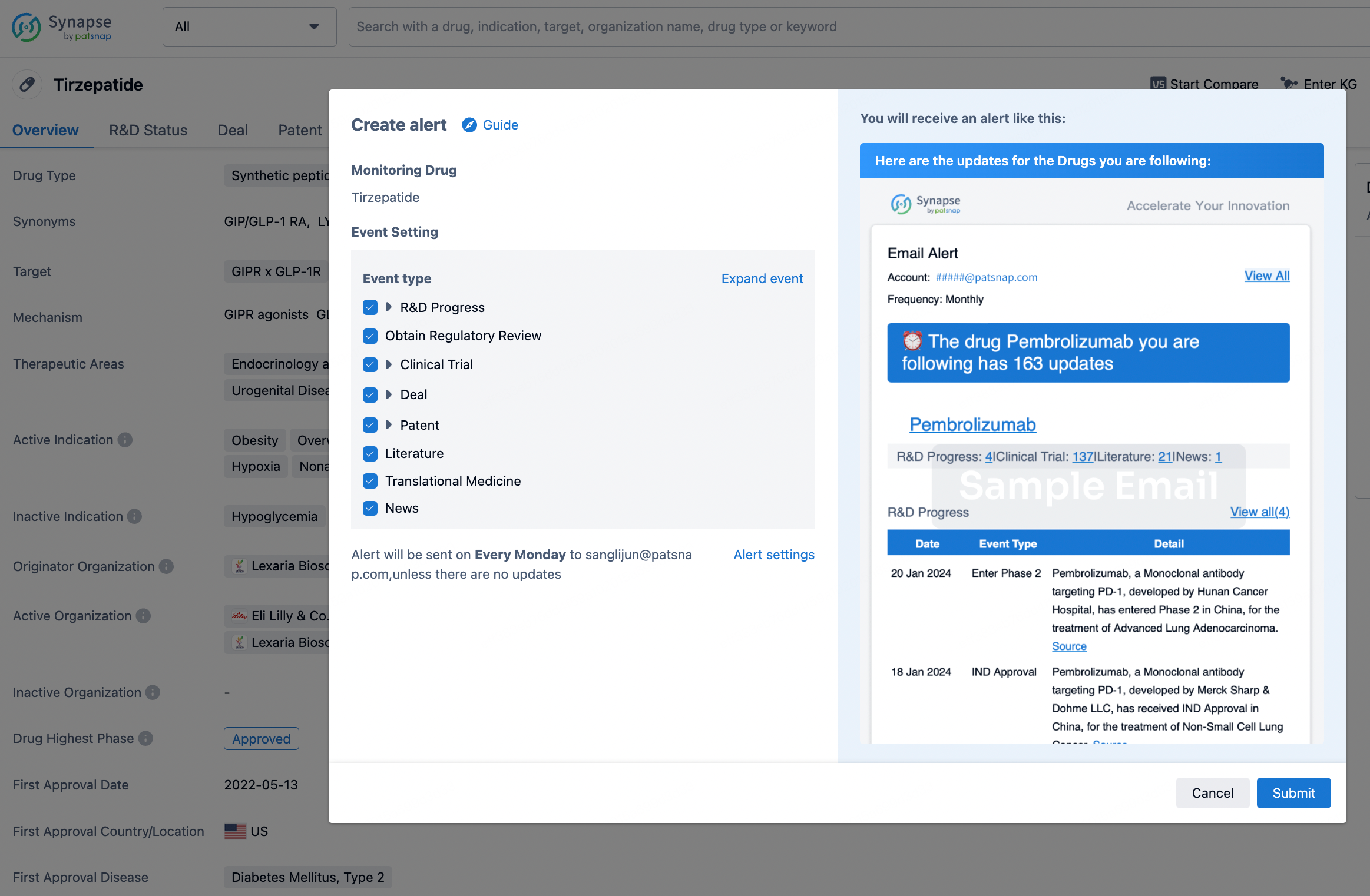
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!