GSK Acquires CMG1A46 from Chimagen Biosciences to Boost Immunology Portfolio
GSK plc (LSE/NYSE: GSK) has entered into an agreement with Chimagen Biosciences (Chimagen), a privately-owned biotech firm, to purchase CMG1A46. This product is a dual-targeted T cell-engager (TCE) that focuses on CD19 and CD20 and is currently in clinical stages. The acquisition is valued at $300 million upfront. GSK intends to advance and market CMG1A46, targeting B cell-mediated autoimmune disorders, including systemic lupus erythematosus (SLE) and lupus nephritis (LN), with the possibility of branching out into other related autoimmune conditions.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
For more than ten years, GSK has led the way in lupus treatment. This partnership highlights the need for innovative therapeutic strategies to tackle the diverse manifestations of lupus and the ongoing challenges faced by patients, especially those with severe forms of the disease who do not respond to conventional treatments.
Tony Wood, GSK's Chief Scientific Officer, stated: “Our research into systemic lupus erythematosus and lupus nephritis has deepened our understanding of the key factors driving B cell-related diseases. CMG1A46, a novel therapeutic that targets intensive B cell depletion, presents an exciting opportunity that we are eager to pursue to meet the unmet needs in lupus and similar autoimmune disorders.”
CD20 has been a well-established target for treating autoimmune conditions, and emerging clinical data suggests that CD19 could be a promising alternative due to its expression on a wider variety of B cell types. In preclinical investigations, CMG1A46, which is engineered to target both CD19 and CD20, has demonstrated rapid and profound B cell depletion in the bloodstream and tissues, potentially resulting in more sustained patient responses.
Zhenhao Zhou, CEO of Chimagen, remarked: “We are enthusiastic about CMG1A46’s potential to enhance the quality of life for individuals with autoimmune diseases and appreciate GSK's role in advancing that vision. This agreement further validates our proprietary T cell-engager platform, and we look forward to continuing our pursuit of innovative multi-specific antibody therapies.”
Currently, CMG1A46 is undergoing phase I clinical trials for leukemia and lymphoma in both the United States and China, with GSK planning to initiate a phase I trial for lupus in 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
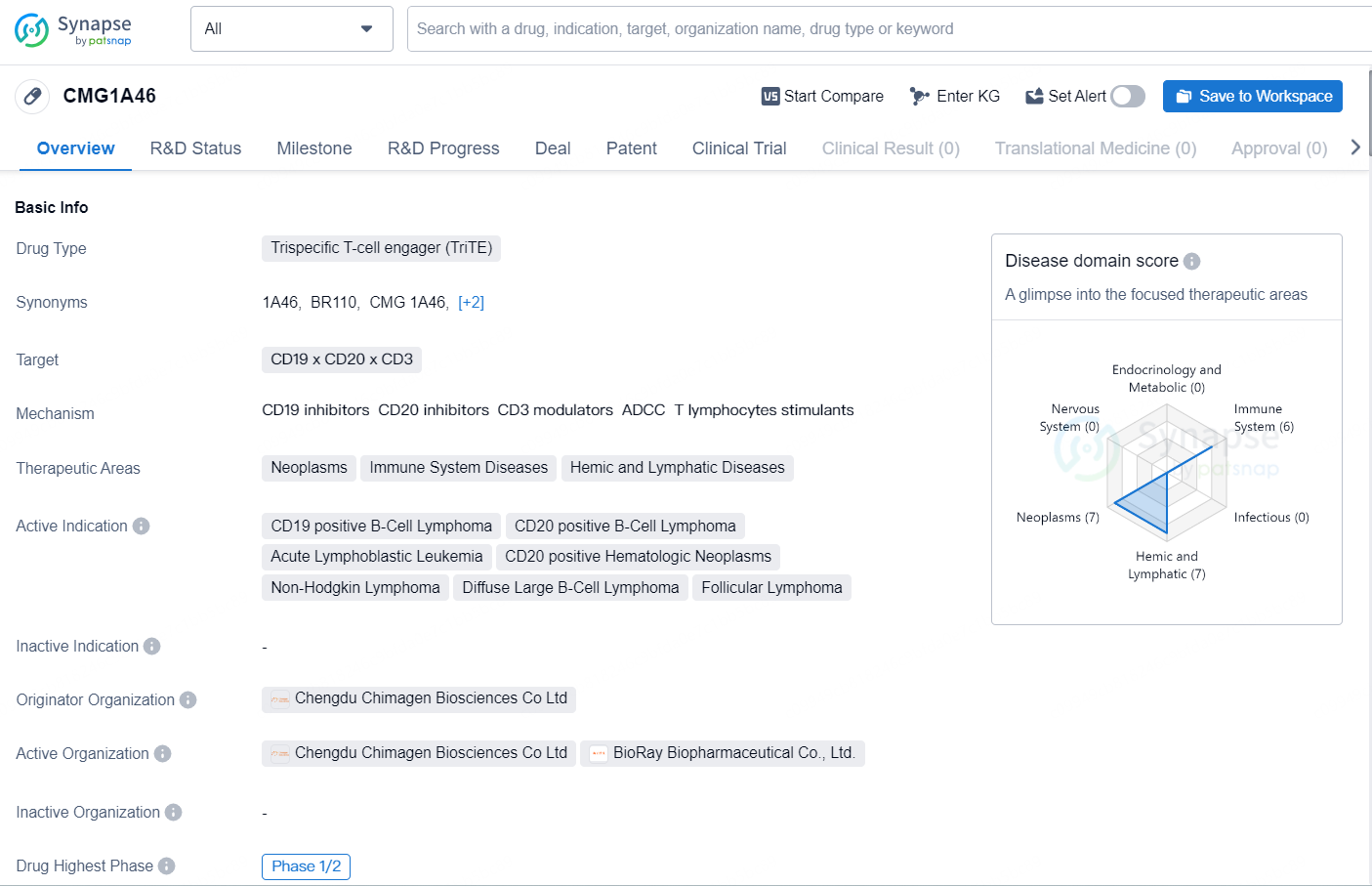
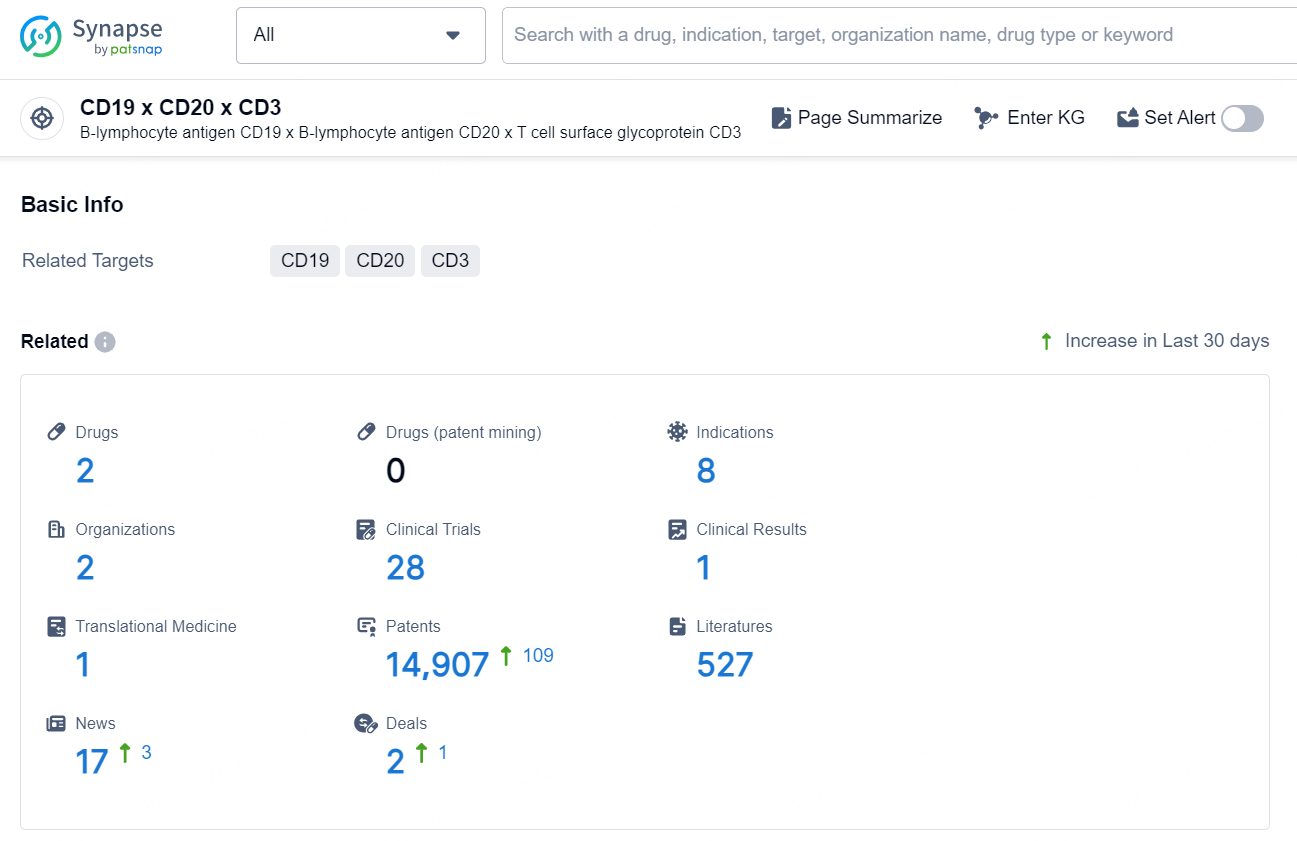
According to the data provided by the Synapse Chemical, As of October 31, 2024, there are 2 investigational drugs for the CD19 x CD20 x CD3 target, including 8 indications, 2 R&D institutions involved, with related clinical trial reaching 28, and as many as 14907 patents.
The drug CMG1A46 is classified as a Trispecific T-cell engager (TriTE) and targets CD19 x CD20 x CD3. It is indicated for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. The active indications of CMG1A46 include CD19 positive B-Cell Lymphoma, CD20 positive B-Cell Lymphoma, Acute Lymphoblastic Leukemia, CD20 positive Hematologic Neoplasms, Non-Hodgkin Lymphoma, Diffuse Large B-Cell Lymphoma, and Follicular Lymphoma.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!