Aro Biotherapeutics Initiates Phase 1b Trial of ABX1100 for Late-Onset Pompe Disease
Aro Biotherapeutics, a biotechnology firm currently in the clinical stage focused on creating effective and tissue-specific therapies, has declared the start of the Phase 1b segment of its clinical trial for ABX1100, an innovative candidate aimed at treating late-onset Pompe disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
ABX1100 targets CD71, a specific receptor, to transport siRNA therapy into muscle tissues, effectively inhibiting the GYS1 enzyme responsible for glycogen synthesis. In the upcoming phase 1b trial, researchers aim to enroll adults diagnosed with late-onset Pompe disease (LOPD) to assess the safety and biological activity of ABX1100.
“The challenges associated with enzyme replacement therapies for Pompe disease highlight the urgent need for innovative treatments with different mechanisms,” remarked Dr. Aneal Khan, the lead investigator of the study and a pediatrician and medical geneticist at the Metabolic and Genetic in Calgary (MAGIC) Clinic in Alberta, Canada. “ABX1100 is under investigation as a potential new treatment option for patients with late-onset Pompe disease, and we are excited to initiate this trial for an underserved group.”
Pompe disease is a rare neuromuscular condition characterized by the harmful accumulation of glycogen, a form of stored sugar, in muscles. This excessive glycogen results in deteriorating muscle function, weakness, disability, and can ultimately lead to death due to respiratory failure. Current enzyme replacement therapies have limited long-lasting effects. Preliminary studies indicate that targeting GYS1 could serve as a beneficial addition or alternative to ERT.
“The positive preclinical and clinical findings accumulated thus far create a robust basis for the continuing clinical investigations of ABX1100, which could represent the first new therapy for Pompe disease beyond ERT. We expect to commence dosing the initial patient with Pompe disease in the Phase 1b study prior to the end of 2024,” stated Susan Dillon, Ph.D., co-founder, president, and CEO of Aro.
“ABX1100 was well tolerated in a recently concluded Phase 1a trial involving healthy volunteers, demonstrating sustained GYS1 mRNA knockdown in muscle tissues, with effects lasting at least 10 weeks post a single administration,” Dr. Dillon elaborated. “The trial successfully established a safe and effective initial dose for subsequent studies in patients with LOPD. We intend to present the findings from the Phase 1a trial at an upcoming medical conference.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
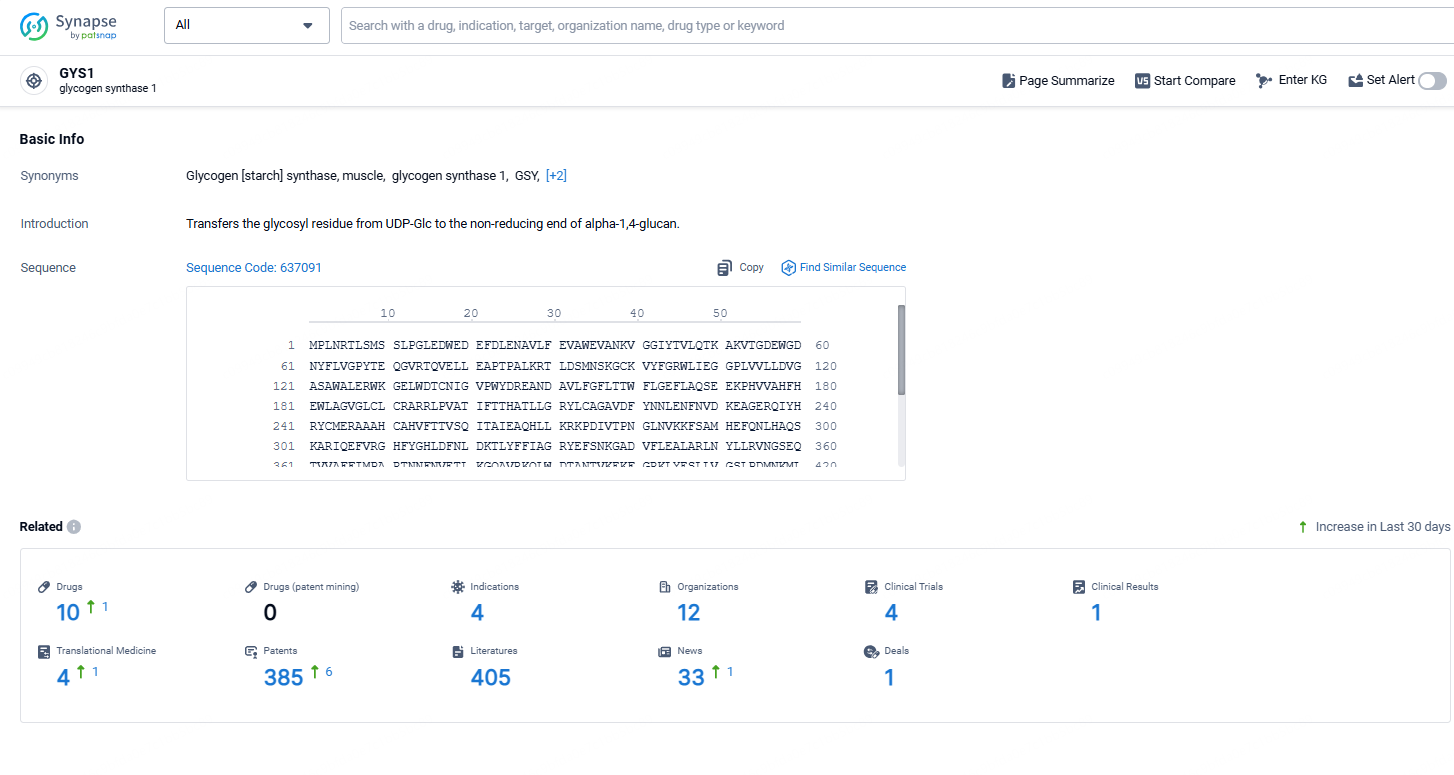
According to the data provided by the Synapse Database, As of October 31, 2024, there are 10 investigational drugs for the GYS1 target, including 4 indication, 12 R&D institutions involved, with related clinical trial reaching 4916, and as many as 385patents.
ABX-1100 shows potential in addressing unmet medical needs in the field of biomedicine, particularly in the treatment of rare genetic disorders such as Glycogen Storage Disease Type II. Its status as an orphan drug underscores the importance of developing treatments for rare and often overlooked conditions. As the drug progresses through clinical development, it will be important to continue monitoring its safety and efficacy profile, as well as its potential impact on the pharmaceutical industry and the healthcare landscape.