Clover's SCB-1019 Shows Promising Results Against GSK's AREXVY in RSV Vaccine Trial
Clover Biopharmaceuticals, Ltd. revealed encouraging supplementary data regarding immunogenicity and safety in senior and elderly participants from its Phase I study of SCB-1019. This candidate is a non-adjuvanted bivalent RSV prefusion-stabilized F (PreF)-Trimer subunit vaccine, developed using Clover's Trimer-Tag vaccine technology platform. The trial involved a direct comparison with GSK’s AS01E-adjuvanted RSV vaccine.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Clover is excited to present favorable head-to-head clinical findings regarding its non-adjuvanted SCB-1019 RSV vaccine candidate in comparison to AS01E-adjuvanted AREXVY, highlighting its promising combined efficacy and safety profile, noted Joshua Liang, Chief Executive Officer and Board Director of Clover.
Liang added, “Although the currently available protein-based RSV vaccines demonstrate safety and effectiveness when administered as the initial dose, there are significant unmet needs globally in terms of effective re-vaccination as protection against RSV diminishes, as well as the prevention of respiratory illnesses caused by other RSV-related viruses. We anticipate assessing SCB-1019 in a re-vaccination context for RSV and as part of a combination vaccine for respiratory illnesses in clinical trials set for 2025.”
In the ongoing Phase I trial, a total of 70 older adult and elderly participants have been recruited to receive either Clover's SCB-1019, GSK's AREXVY, or a saline placebo. Below is a summary of the preliminary findings related to immunogenicity and safety for SCB-1019:
- RSV Neutralizing Antibodies: The non-adjuvanted SCB-1019 generated geometric mean titers of neutralizing antibodies against RSV-A and RSV-B that were similar to those observed with the AS01E-adjuvanted AREXVY at Day 28, showing no statistically significant differences.
- RSV-B Specific Antibodies: SCB-1019 exhibited an approximately 1.5-fold higher trend in antibodies targeting a potent RSV-B specific neutralization epitope at Site V compared to AREXVY, according to an exploratory competitive-ELISA assay, suggesting the potential for enhanced and longer-lasting immune response upon re-vaccination if validated in further studies.
In light of these encouraging results from the Phase I trial, Clover intends to commence clinical trials in 2025, investigating SCB-1019 (a non-adjuvanted bivalent RSV-A/B vaccine candidate) in the context of RSV re-vaccination and as part of a combination vaccine for respiratory diseases.
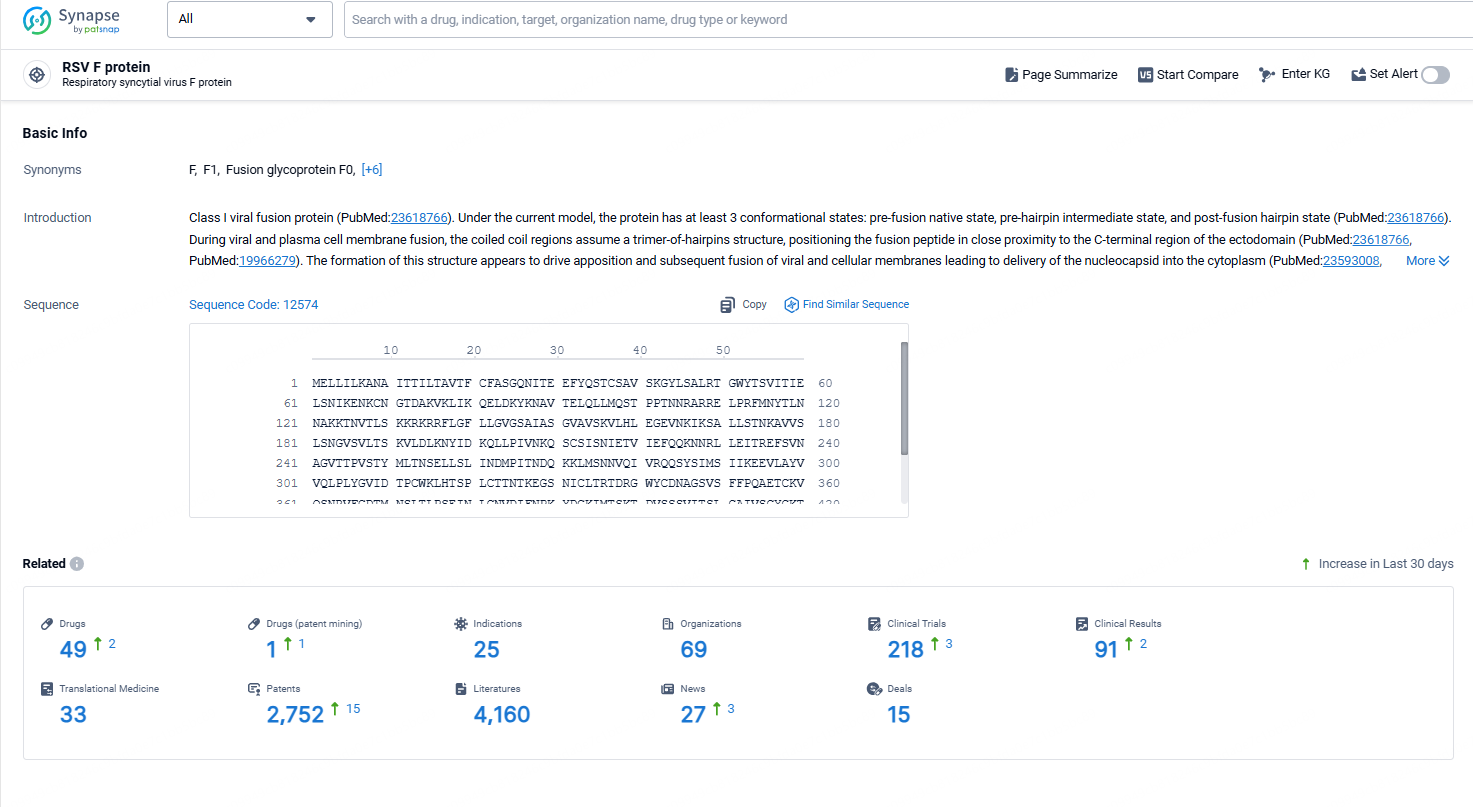
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 31, 2024, there are 49 investigational drugs for the RSV F protein target, including 25 indications, 69 R&D institutions involved, with related clinical trial reaching 218, and as many as 2752 patents.
The RSV F antigen vaccine, developed by Clover Biopharmaceuticals, is a vaccine targeting the RSV F protein associated with respiratory syncytial virus (RSV) infections. It is important to note that while the RSV F antigen vaccine shows promise in its early development stages, further research and clinical trials will be necessary to assess its safety and efficacy in larger populations. Additionally, regulatory approval will be required before the vaccine can be made available to the public.