GSK's PD-1 inhibitor Dostarlimab combined with chemotherapy for the treatment of dMMR endometrial cancer receives FDA approval
On August 1, 2023, GlaxoSmithKline (GSK) announced that the US FDA has approved its PD-1 inhibitor Jemperli (Dostarlimab) to be combined with carboplatin and paclitaxel, and to continue Jemperli as a single agent to treat adult patients with primary advanced or recurrent endometrial cancer identified as mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H) using an FDA-approved diagnostic method. Dostarlimab is the first immunotherapy approved by the FDA to be used in combination with chemotherapy for the first-line treatment of this patient population.
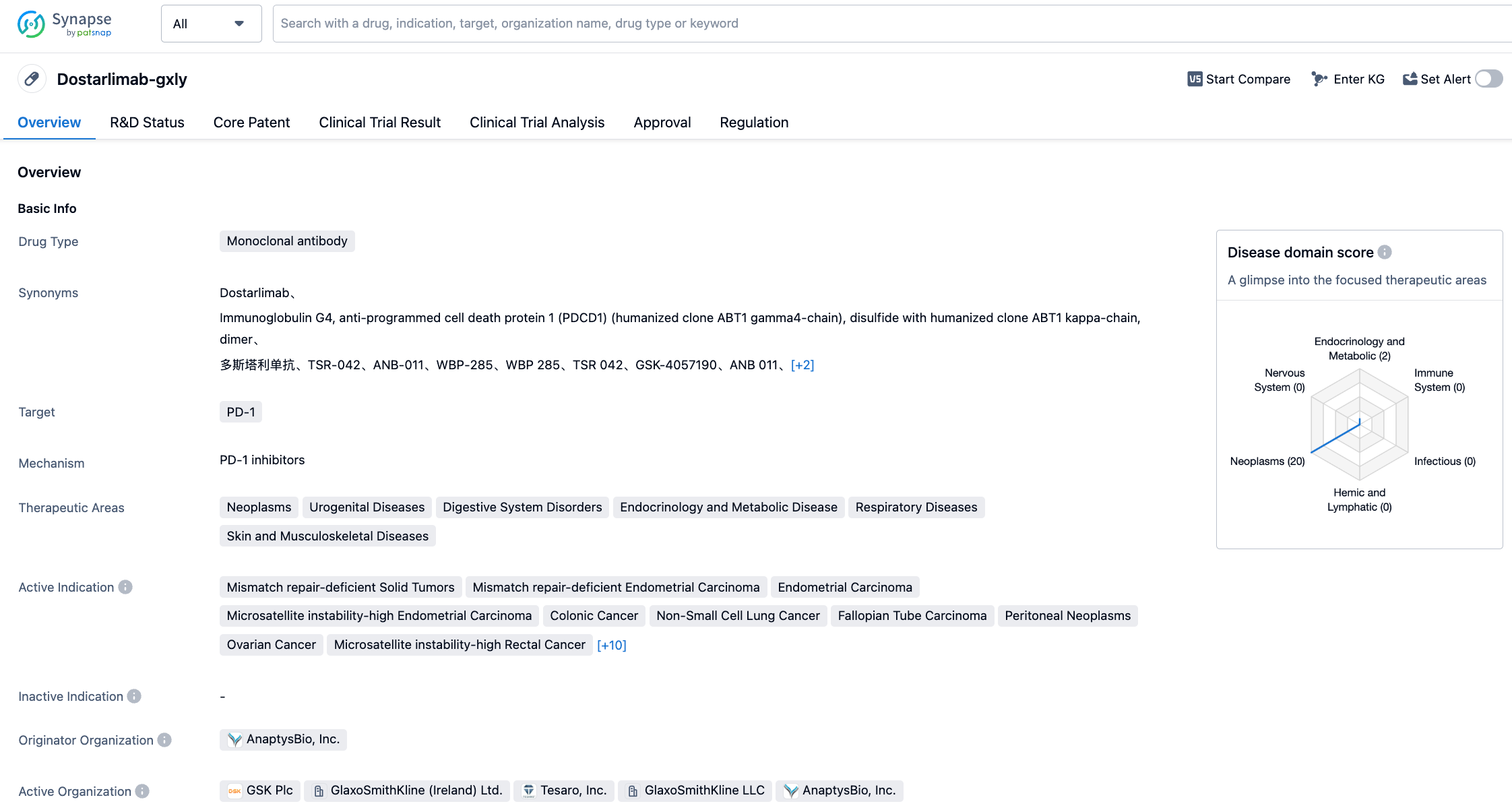
Dostarlimab (Jemperli) is a PD-1 monoclonal antibody from GSK that can bind to the PD-1 receptor and block its interaction with ligands PD-L1 and PD-L2. In April 2021, the FDA granted accelerated approval for Dostarlimab to treat adults with recurrent or advanced endometrial cancer who have DNA mismatch repair deficiencies (dMMR) and have progressed after platinum-based chemotherapy. In August 2021, the FDA granted accelerated approval for a new indication for Dostarlimab to treat patients with dMMR recurrent or advanced solid tumors. With the FDA's approval for the new indication for Dostarlimab combined with chemotherapy to treat adult patients with primary advanced or recurrent endometrial cancer with dMMR, it is expected to expand the commercial footprint of Dostarlimab.
This approval is primarily based on interim analysis results from part 1 of the phase 3 RUBY trial, with a median follow-up duration of ≥25 months. The results were recently presented at the 2023 American Society of Clinical Oncology (ASCO) annual meeting. Overall, based on the assessment by the blinded independent central review (BICR), results showed that Dostarlimab in combination with the standard chemotherapy of carboplatin and paclitaxel showed better efficacy than the combination with placebo. The analysis showed that one primary endpoint was achieved, namely a statistically and clinically significant improvement in progression-free survival (PFS) as assessed by the investigators in the dMMR/MSI-H patient population treated with Jemperli plus carboplatin and paclitaxel. In addition, a 71% reduction in the risk of disease progression or death was observed in dMMR/MSI-H patients receiving this combination therapy. The safety and tolerability characteristics of the Jemperli plus chemotherapy combination were consistent with the known safety profiles of the individual drugs. In patients receiving combination therapy, the most common adverse events (≥20%) occurring after treatment were rash, diarrhea, hypothyroidism, and hypertension.
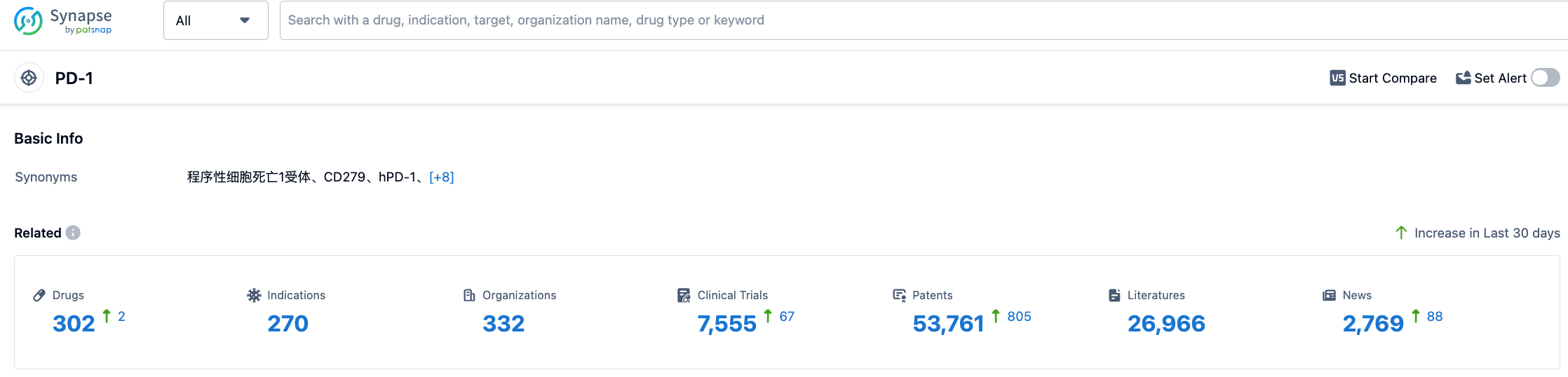
According to the information disclosed by Synapse (click to access the PD-1 target after registration and login to obtain free details of the drugs under research, indications, research institutions, clinical trials, and patents under this target), there are 302 PD-1 targeted drugs under research, covering 270 indications, 332 research institutions, involving 7550 related clinical trials, and up to 53721 patents as of August 2, 2023. We look forward to the future performance of Dostarlimab.