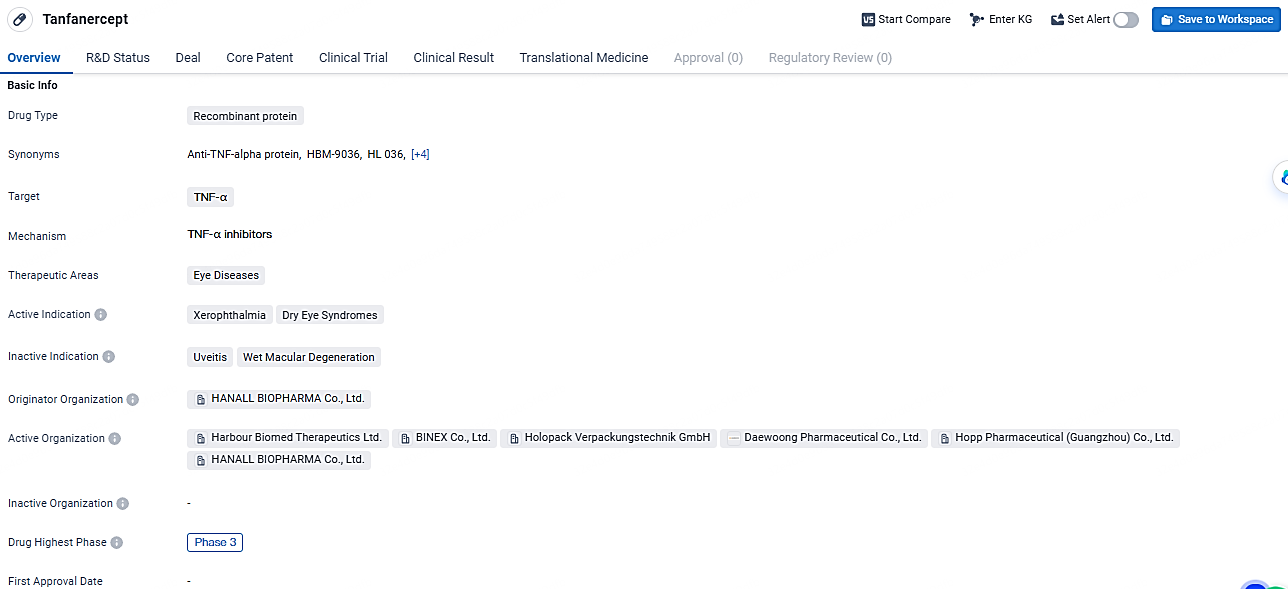
HanAll Biopharma Initiates Phase III VELOS-4 Trial of Tanfanercept for Dry Eye Syndrome
HanAll Biopharma Co., Ltd., an international biopharmaceutical firm focused on the creation and advancement of new treatments for patients, has disclosed the commencement of its Phase III VELOS-4 study. This trial aims to assess the effectiveness and safety of tanfanercept, an innovative, topical anti-inflammatory medication, in subjects suffering from moderate to severe dry eye disease.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Chronic dry eye disease affects about 14.5% of people in the United States and is characterized by multiple causal factors. Current treatment options for dry eye disease (DED) often fall short, occasionally leading to visual problems and inflammation of the eye, which can result in poor patient adherence due to low efficacy.
One novel approach in addressing this condition is Tanfanercept, an advanced first-of-its-kind topical medication aimed at reducing inflammation. This treatment specifically targets the tumor necrosis factor (TNF), a crucial inflammatory cytokine in DED, and is being developed in collaboration with Daewoong Pharmaceutical. Tanfanercept utilizes a recombinant fragment of tumor necrosis factor receptor 1 designed to bind strongly to TNF and is engineered to be stable against proteinase degradation.
Significant progress was made in the Phase III VELOS-4 trial, building on the completed Phase III VELOS-3 study. Results from VELOS-3 indicated that tanfanercept significantly enhanced tear production as measured by the unanesthetized Schirmer test at eight weeks for patients who received tanfanercept versus those who received a placebo.
Additionally, post hoc findings from VELOS-3 showed that a substantial number of participants treated with tanfanercept achieved a Schirmer test increase of 10mm or more from the baseline at eight weeks, a result which was statistically significant when compared to the placebo group. Earlier, the VELOS-2 study showed similar beneficial effects based on Schirmer test results in a similarly structured analysis.
According to the 2020 FDA Draft Guidance for Developing Drugs for Dry Eye Disease, having participants show a minimum improvement of 10mm in Schirmer test scores is an approved primary efficacy measure for regulatory submission.
Sean Jeong, M.D., MBA, CEO of HanAll Biopharma, commented, “Starting the Phase III VELOS-4 trial is a crucial step forward in our quest to meet the unresolved needs of dry eye patients. Considering the encouraging outcomes from prior studies and the unique TNF-targeting mechanism of tanfanercept, we are optimistic about its potential to bring substantial relief to individuals struggling with this challenging disease.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
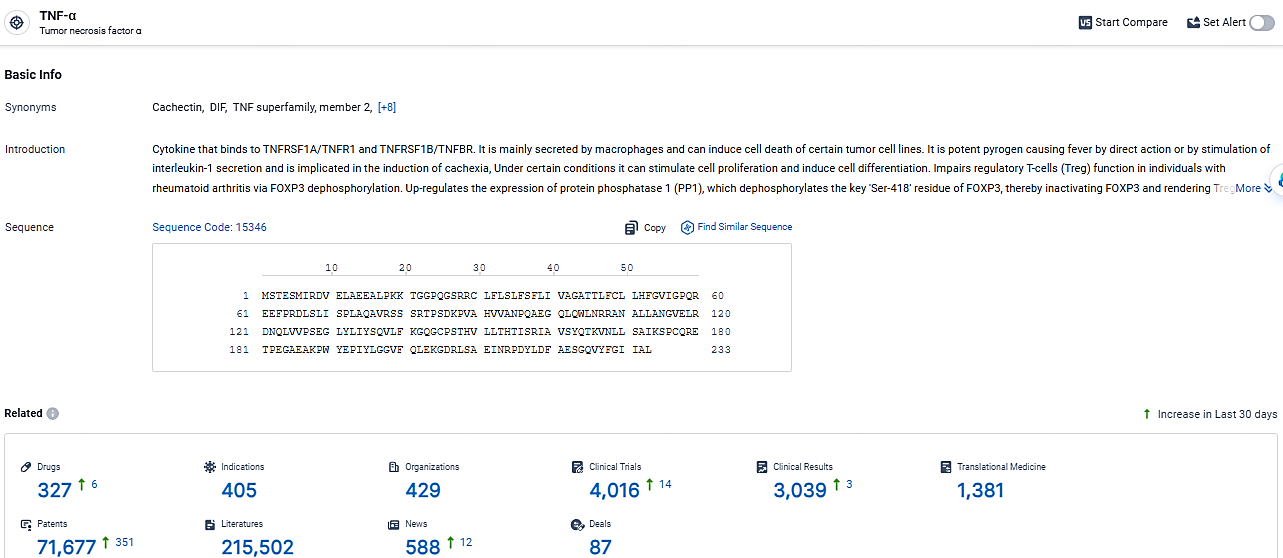
According to the data provided by the Synapse Database, As of May 6, 2024, there are 327 investigational drugs for the TNF-α targets, including 405 indications, 429 R&D institutions involved, with related clinical trials reaching 4016, and as many as 71677 patents.
Tanfanercept targets TNF-α and is primarily used in the treatment of eye diseases, specifically xerophthalmia and dry eye syndromes. With its highest phase being Phase 3, Tanfanercept is currently undergoing advanced clinical trials to assess its safety and efficacy.