HEMGENIX® Gene Therapy Used on First Hemophilia B Patients in Europe by CSL Behring
Leading the field in biotechnology, CSL Behring reported that two individuals with hemophilia B received the gene therapy HEMGENIX (etranacogene dezaparvovec) at Hemophilia Treatment Centers located in France. This significant breakthrough designates HEMGENIX as the pioneering gene therapy to be utilized as a treatment for hemophilia B in a practical, real-world context within Europe.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
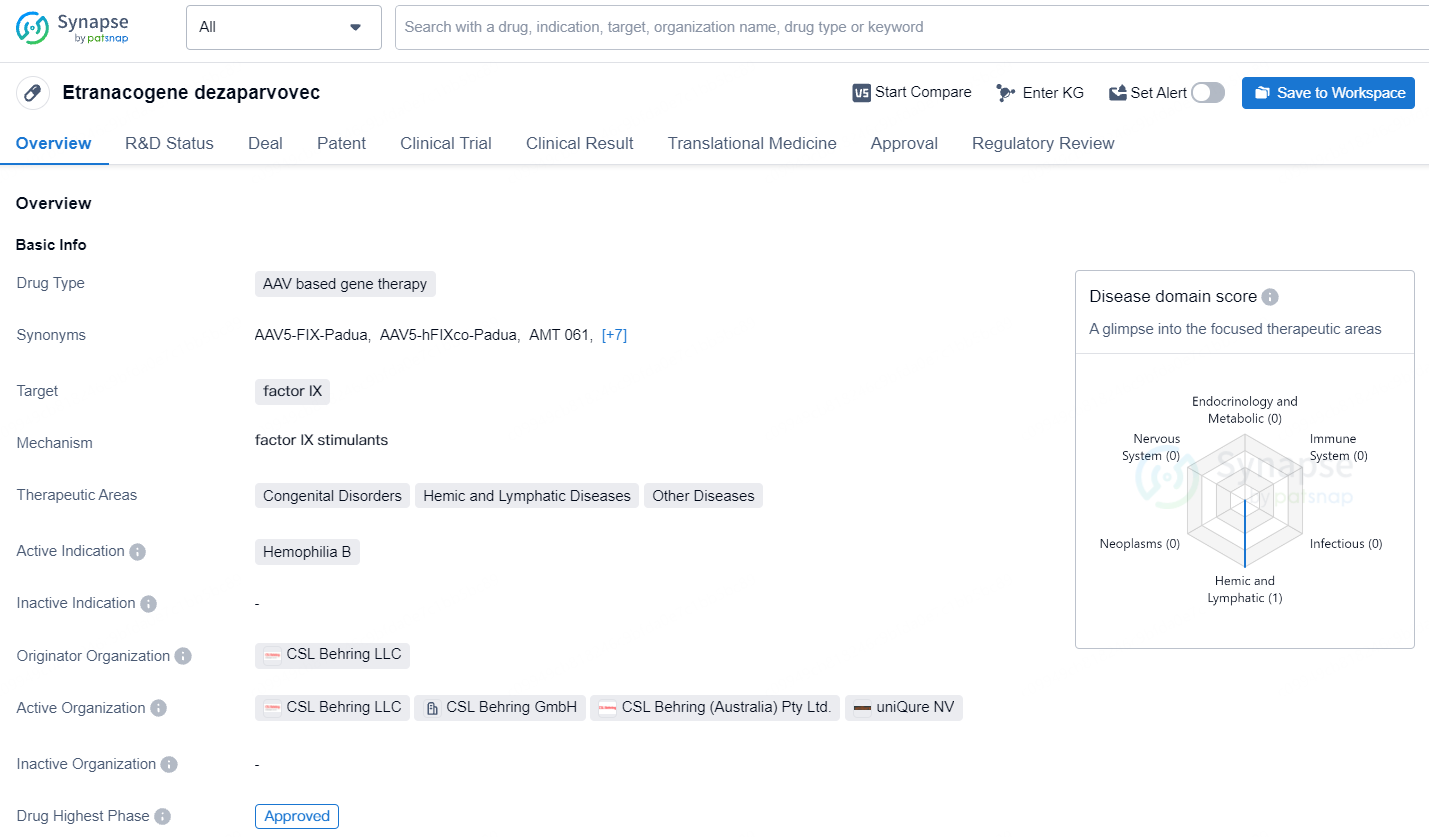
HEMGENIX is the inaugural one-time gene therapy sanctioned in Europe for treating adults with severe and moderately severe hemophilia B, a genetic bleeding disorder due to a deficiency in Factor IX. It is specifically for use in adults who do not have a history of Factor IX inhibitors.
Following its approval by the European Commission, HEMGENIX became the first therapy to achieve Direct Access status in France, permitting the initial cohort of patients in Europe to be treated outside clinical trials.
Current treatments, though effective, can be resource-intensive and demand regular administration, significantly affecting a patient’s everyday life. In contrast, HEMGENIX provides a single administration option, enabling individuals with hemophilia B to generate their own Factor IX, thereby potentially reducing bleeding occurrences.
"Just a few decades ago, the idea of gene therapy for hemophilia seemed futuristic, yet it is now a tangible reality. The treatment of the first two patients with HEMGENIX after receiving European approval marks a significant achievement and reflects the collective efforts of the hemophilia B community and regulatory authorities to introduce groundbreaking treatments to patients," stated Dr. Lutz Bonacker, SVP and General Manager, CSL Behring Commercial Operations Europe.
"This important milestone has been facilitated by the innovative Direct Access policy in France, allowing patients early access to cutting-edge treatments. We are heartened by the increasing availability of gene therapies in European countries and remain dedicated to ensuring ongoing access to potentially transformative treatments," added Dr. Lutz Bonacker, SVP.
HEMGENIX received conditional marketing authorization from the European Commission for the European Union and European Economic Area in February 2023, following its approval by the U.S. Food and Drug Administration in November 2022. It has also been sanctioned by Health Canada, the United Kingdom’s Medicines and Healthcare products Regulatory Agency, Switzerland’s Swissmedic, and Australia’s Therapeutic Goods Administration.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
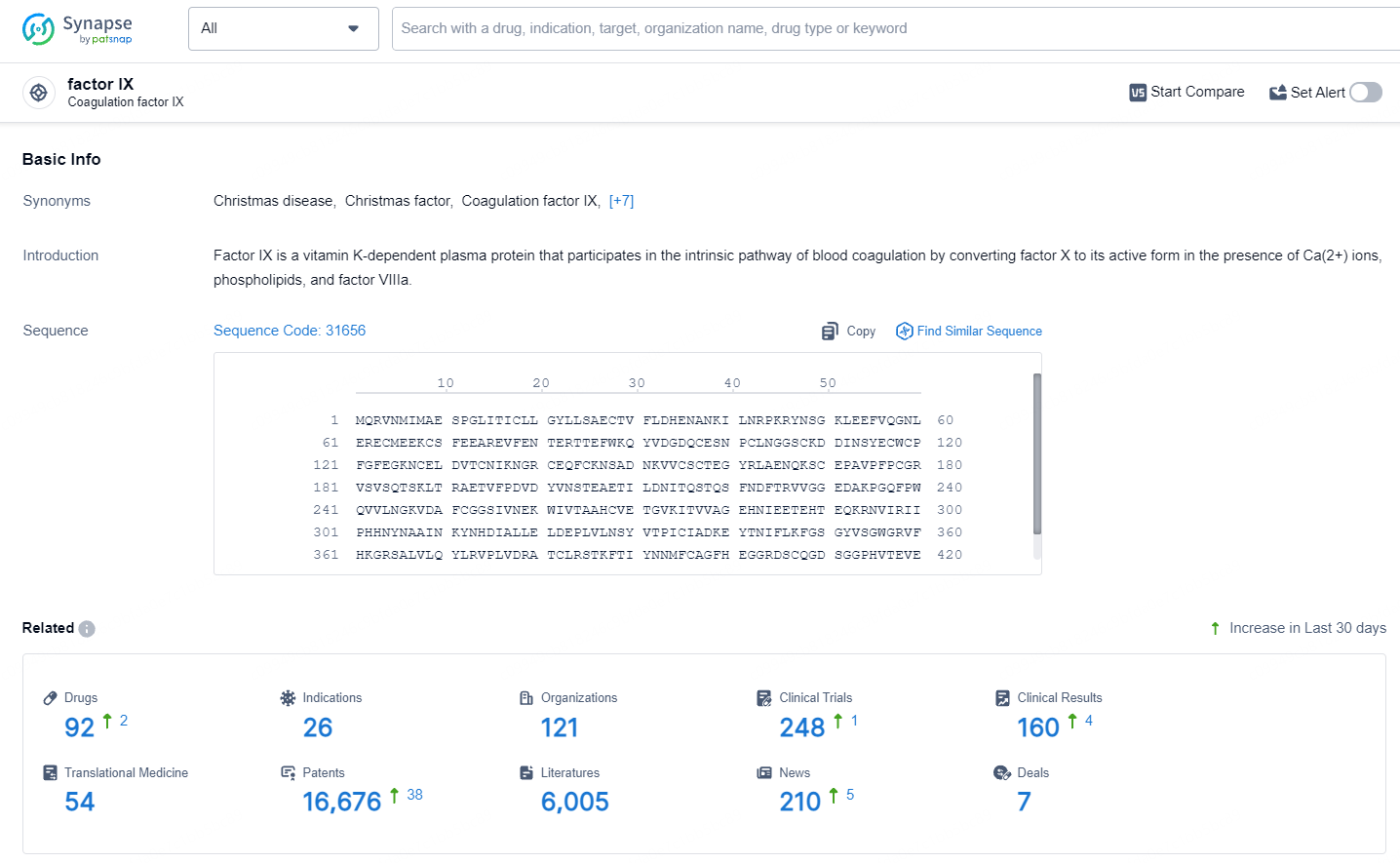
According to the data provided by the Synapse Database, As of July 9, 2024, there are 92 investigational drugs for the Factor IX target, including 26 indications, 121 R&D institutions involved, with related clinical trials reaching 248, and as many as 16676 patents.
Etranacogene dezaparvovec is designed to target factor IX and is indicated for the treatment of Hemophilia B, a congenital disorder that affects the blood's ability to clot. Etranacogene dezaparvovec represents a promising development in the pharmaceutical industry, particularly in the area of gene therapy for genetic disorders. Its approval underscores the growing importance of innovative treatment modalities and the potential for significant advancements in addressing unmet medical needs.