Is Margetuximab approved by the FDA?
Margetuximab, sold under the brand name Margenza, is a medication used in combination with other cancer drugs to treat HER2-positive breast cancer that has metastasized, or spread to other parts of the body. Margetuximab was approved by the US Food and Drug Administration (FDA) on December 16, 2020, for the treatment of metastatic HER2-positive breast cancer in combination with chemotherapy.
Uses and Administration
Uses:
- Margetuximab is used to treat HER2-positive breast cancer that has metastasized.
- It is indicated for patients who have previously received two or more anti-HER2 regimens, with at least one regimen for metastatic disease.
Administration:
- Margetuximab is administered as an intravenous infusion.
- The typical dosage is 15 mg/kg, given once every three weeks.
- The initial infusion is given over 120 minutes, and subsequent doses are administered over 30 minutes.
- The treatment continues until disease progression or unacceptable toxicity occurs.
- Heart function should be monitored using an electrocardiograph (EKG) or ultrasound during the course of treatment.
Side Effects
Common Side Effects:
- Feeling weak or tired
- Stomach pain, nausea, vomiting, loss of appetite
- Diarrhea, constipation
- Fever
- Hair loss
- Headache
- Cough, trouble breathing
- Pain, numbness, or tingling in arms or legs
- Muscle or joint pain
- Hand-foot syndrome (pain, blisters, severe rash on palms or soles)
- Side effects during the infusion
Serious Side Effects:
- New or worsening cough
- Blisters or ulcers in the mouth, red or swollen gums, trouble swallowing
- Heart problems such as pounding heartbeats, dizziness, swelling in the face or lower legs, rapid weight gain, shortness of breath
- Low white blood cell counts leading to fever, mouth sores, skin sores, sore throat, cough, trouble breathing
If any severe side effects occur, contact your doctor immediately. You can report side effects to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Margetuximab may cause heart problems. Symptoms to watch for include fast or pounding heartbeats, cough, shortness of breath, swelling, or rapid weight gain.
- Patients should inform their doctor if they have ever had heart problems.
- A negative pregnancy test may be required before starting treatment, and effective birth control should be used during treatment and for at least four months after the last dose.
- Breastfeeding is not recommended while using margetuximab and for at least four months after the last dose.
Conclusion
This targeted therapy provides a new option for managing advanced breast cancer, offering hope to patients who have exhausted other treatment options. As with any medication, it is important to follow the prescribed instructions and consult healthcare providers with any concerns.
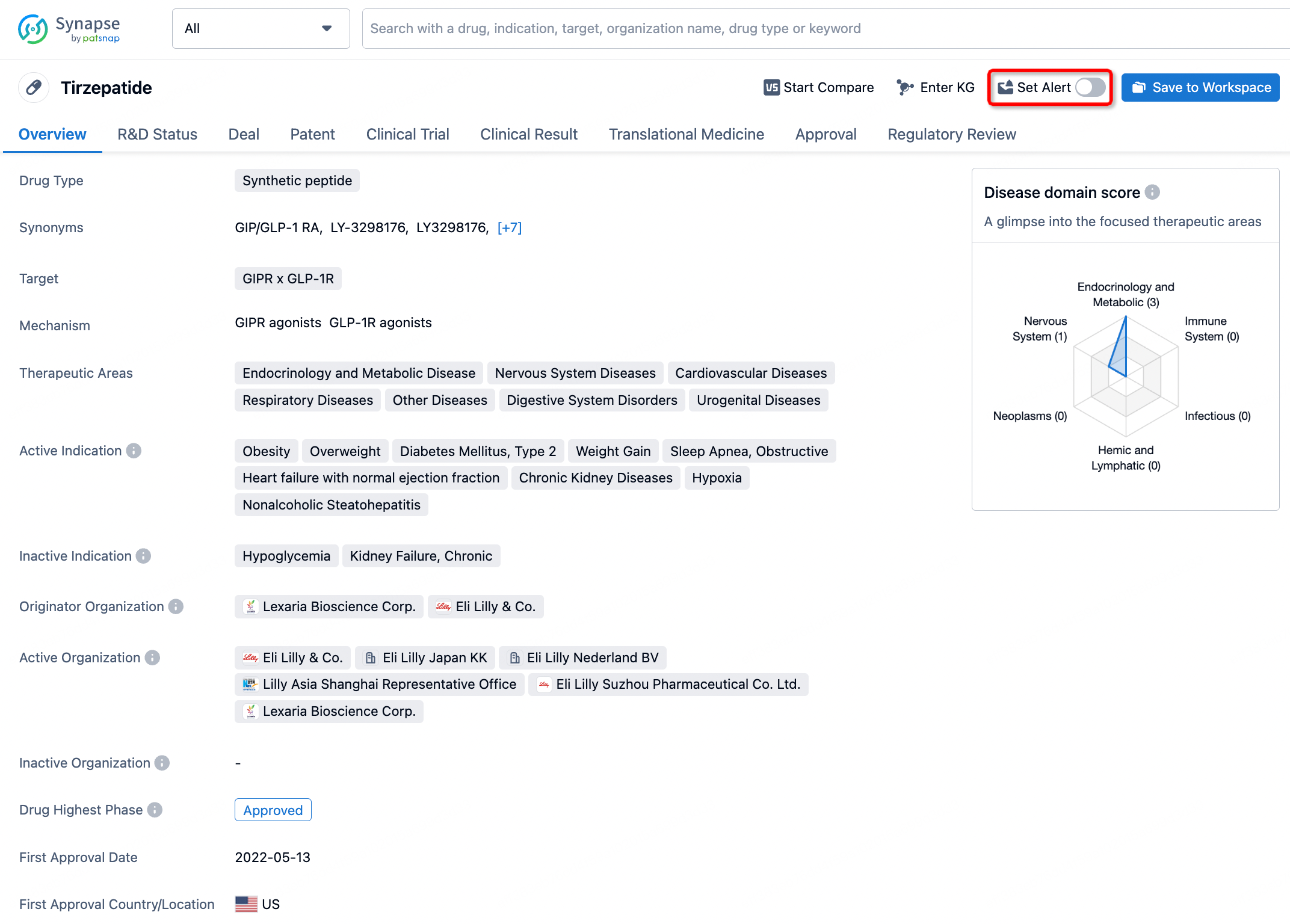
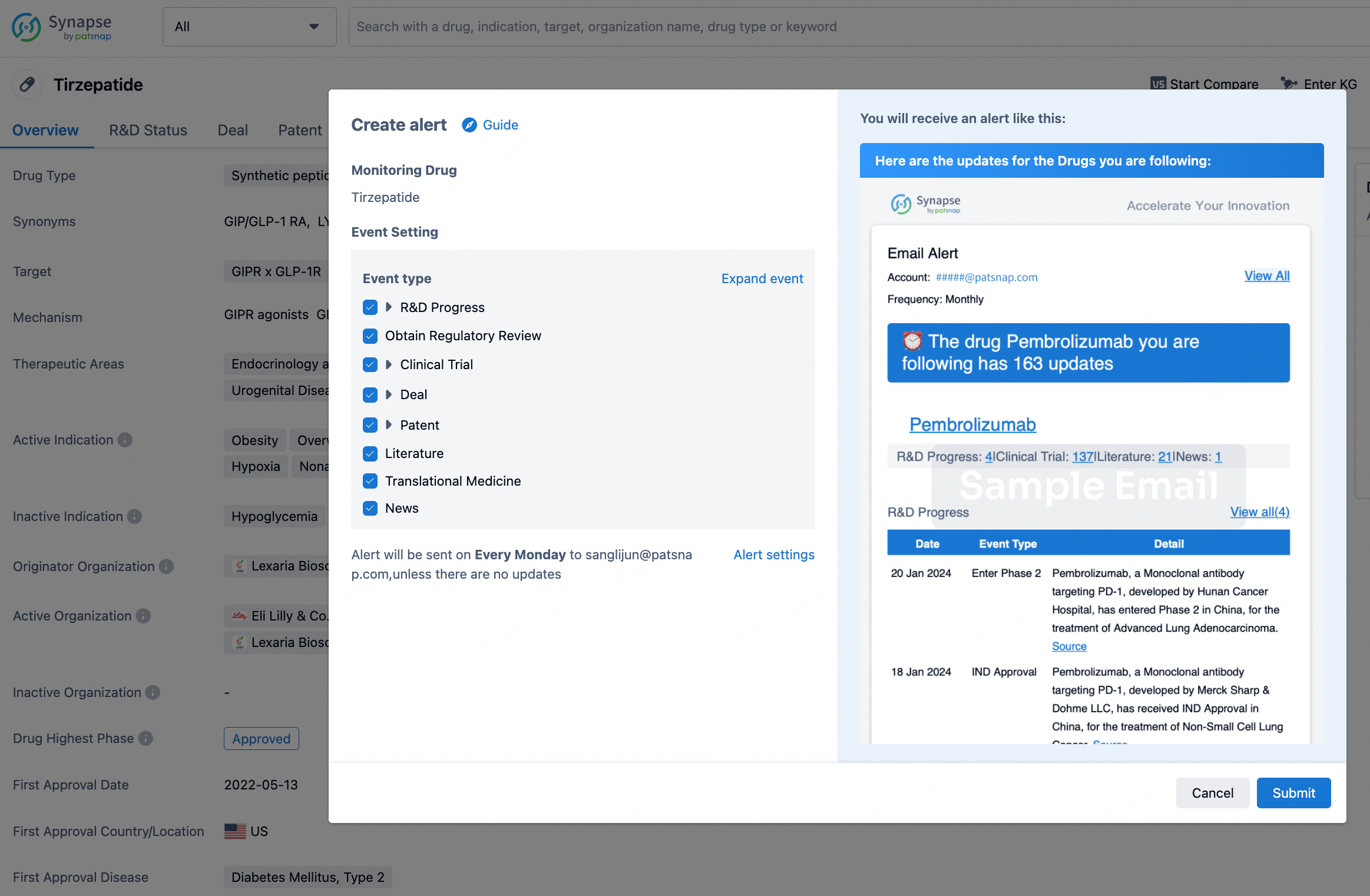
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!