How to find the chemical modification of fitusiran?
Fitusiran is an investigational RNA interference (RNAi) therapeutic developed by Alnylam Pharmaceuticals in collaboration with Sanofi Genzyme. It is designed to target antithrombin (AT) in the liver for the treatment of hemophilia A and B, with or without inhibitors. As a small interfering RNA (siRNA) therapy, fitusiran represents a novel approach to addressing the unmet needs in hemophilia management by rebalancing the coagulation cascade through AT reduction.
Summary of Research Progress of fitusiran
The development of fitusiran stems from the growing understanding of RNAi mechanisms and their potential therapeutic applications. Alnylam Pharmaceuticals, a pioneer in RNAi therapeutics, has leveraged its expertise in siRNA design and delivery to create fitusiran as a subcutaneously administered drug that can potentially offer long-lasting effects with less frequent dosing compared to traditional factor replacement therapies.
Fitusiran's mechanism of action is based on silencing the SERPINC1 gene, which encodes for antithrombin, a potent natural anticoagulant. By reducing AT levels, fitusiran aims to enhance thrombin generation and improve hemostasis in patients with hemophilia, regardless of the underlying factor deficiency or the presence of inhibitors2. This approach is particularly promising for patients with inhibitors, who often have limited treatment options and face challenges with current bypassing agents.
The global development of fitusiran has progressed through various clinical trial phases. As of the latest available information, fitusiran is not yet approved or listed in any country but is undergoing phase 3 clinical trials. The global competition landscape for hemophilia treatments is evolving rapidly, with several novel approaches being investigated alongside fitusiran. These include other RNAi therapies, bispecific antibodies like emicizumab, and gene therapies.
Clinical research status for fitusiran has been marked by both promising results and safety concerns. Phase 1 and 2 studies demonstrated the drug's potential to significantly reduce bleeding events in hemophilia patients. However, the development program has faced challenges, including a temporary clinical hold in 2017 due to a fatal thrombotic event. This led to the implementation of additional safety measures and protocol amendments.
The ATLAS phase 3 clinical trial program for fitusiran includes several studies evaluating its efficacy and safety in different hemophilia populations. The ATLAS-INH and ATLAS-A/B trials have shown significant reductions in annualized bleeding rates compared to on-demand factor therapy or bypassing agents. In the ATLAS-PPX study, fitusiran demonstrated superiority over prior factor or bypassing agent prophylaxis in reducing bleeding episodes.
Sequence Information and Characteristics of fitusiran
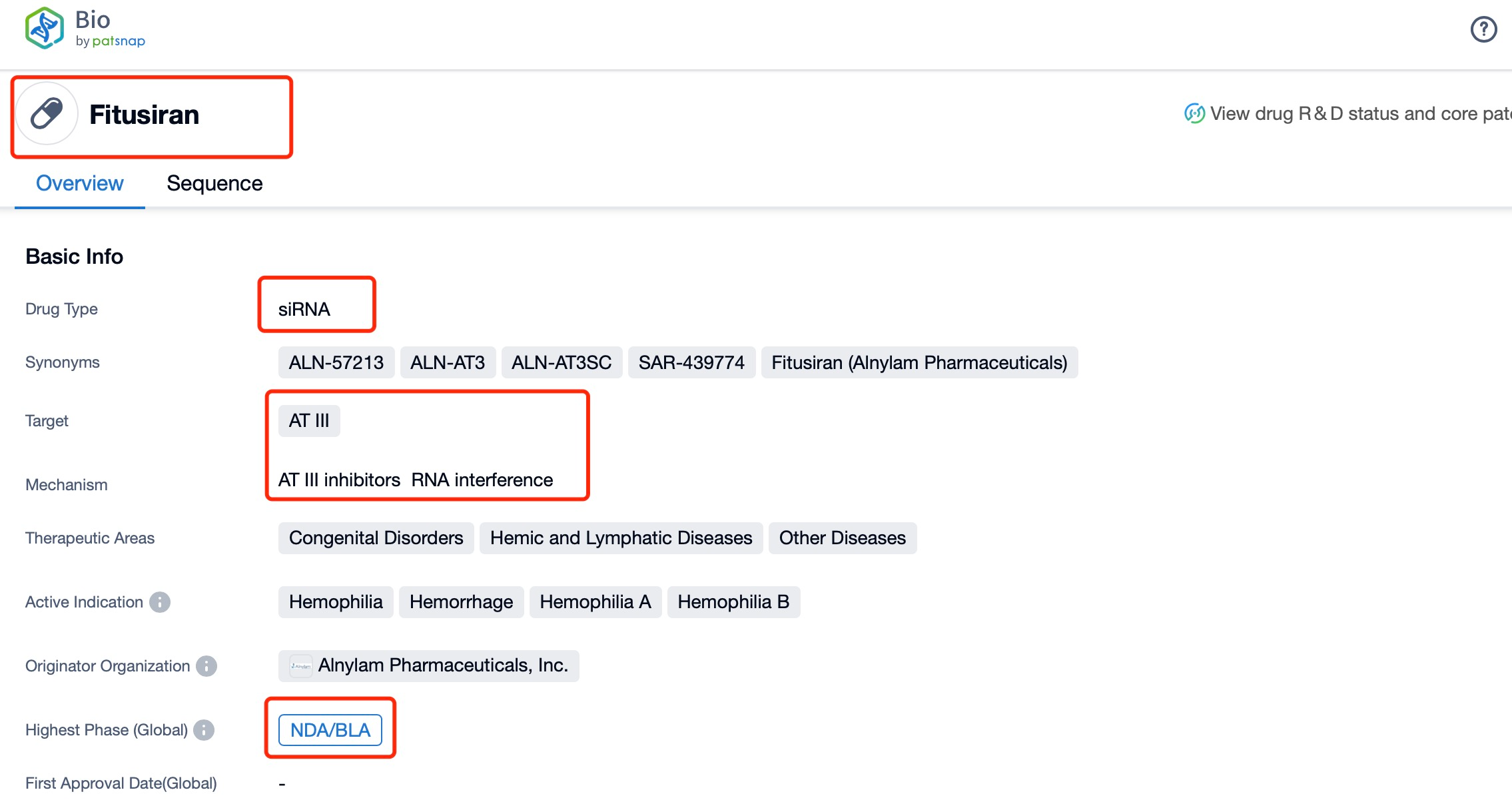
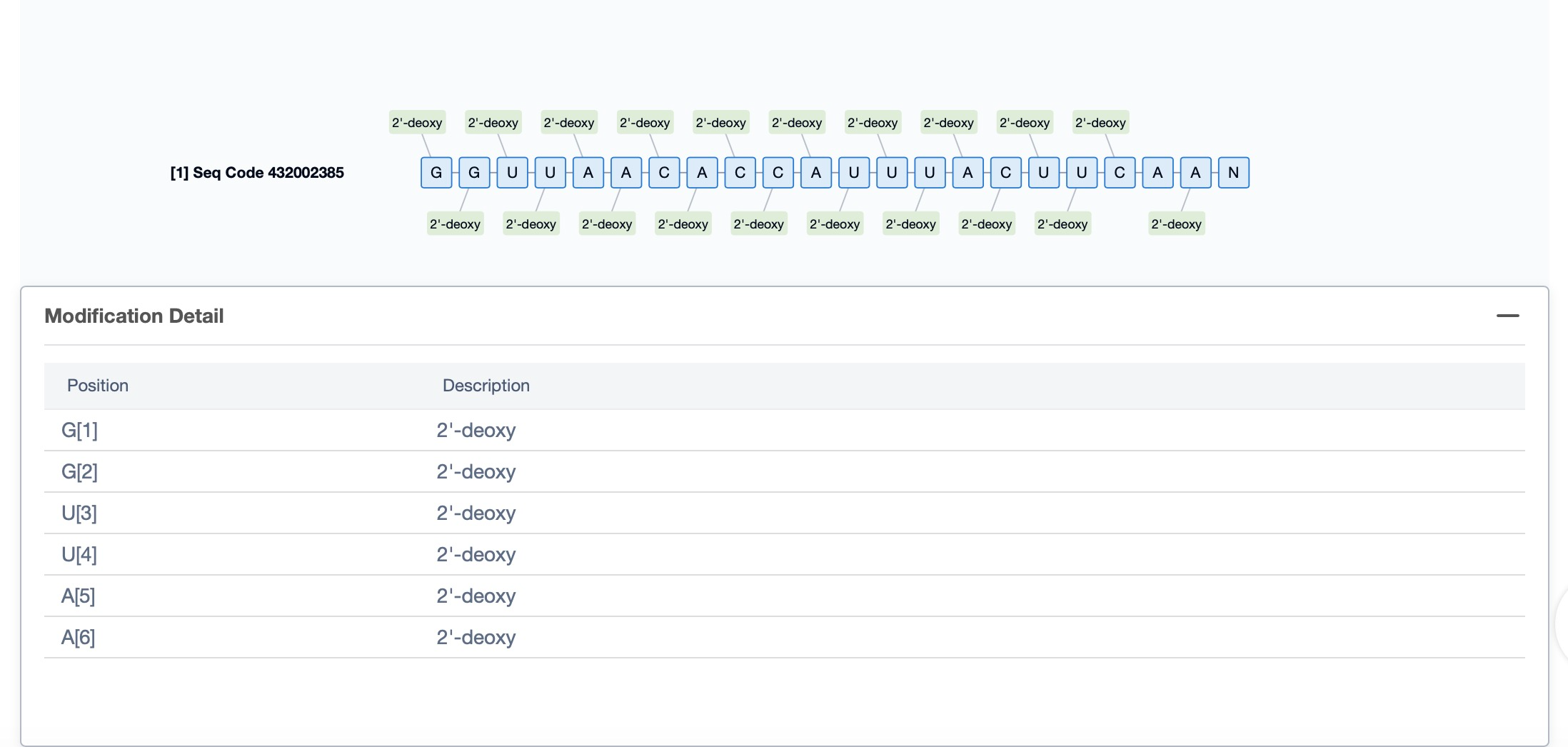
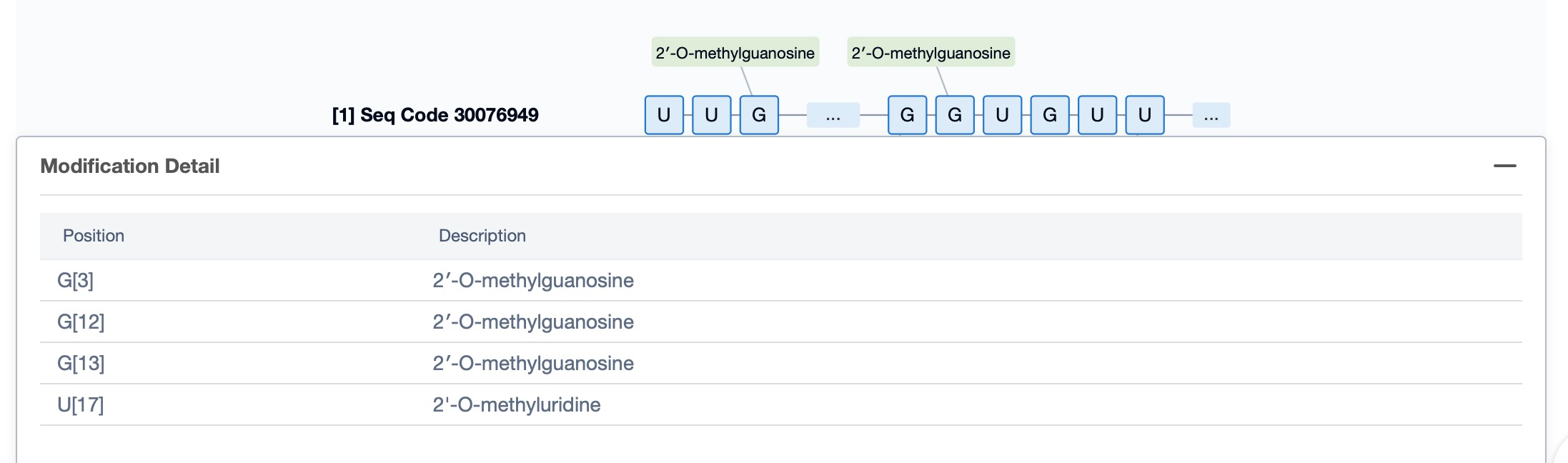
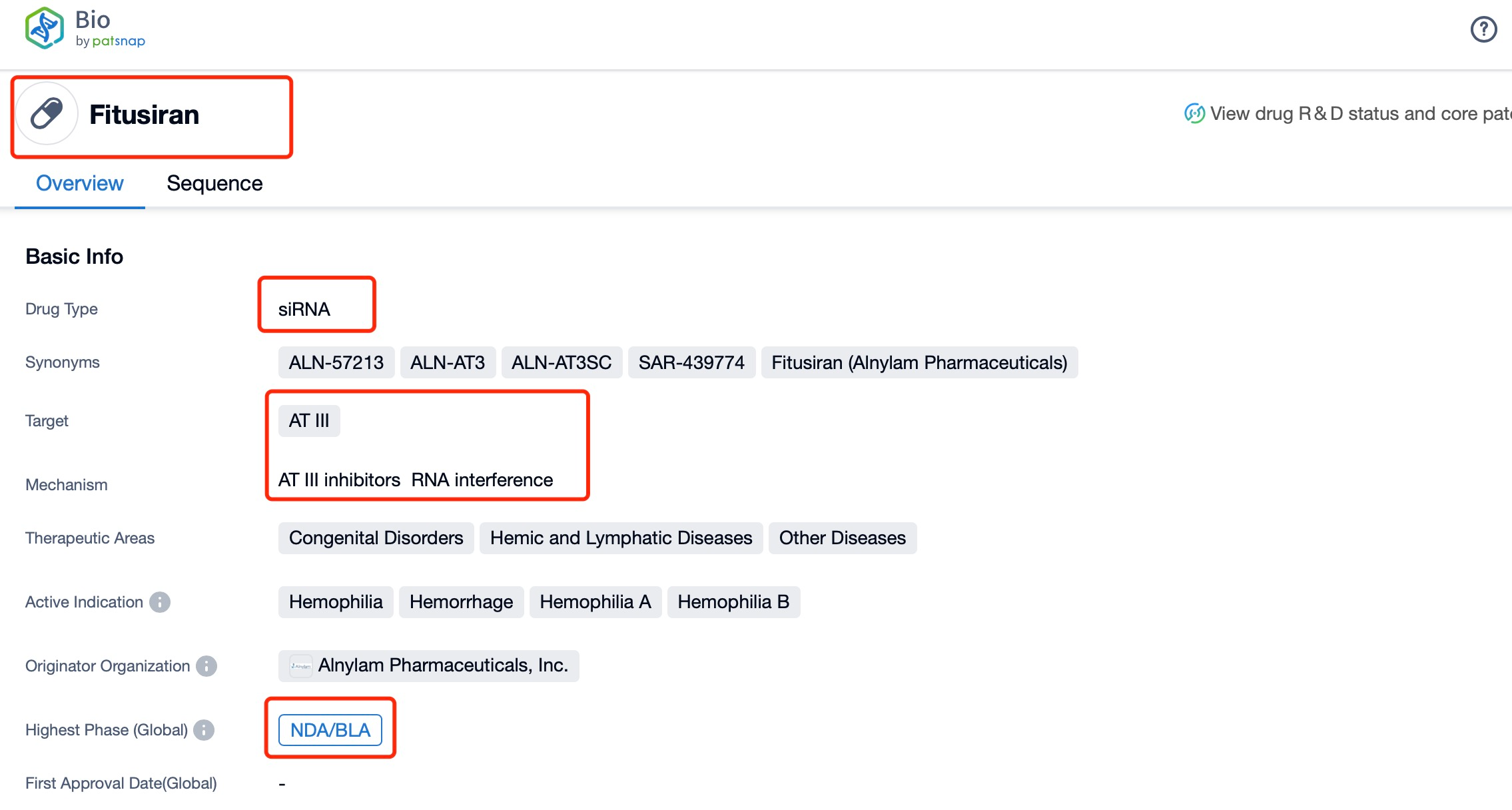
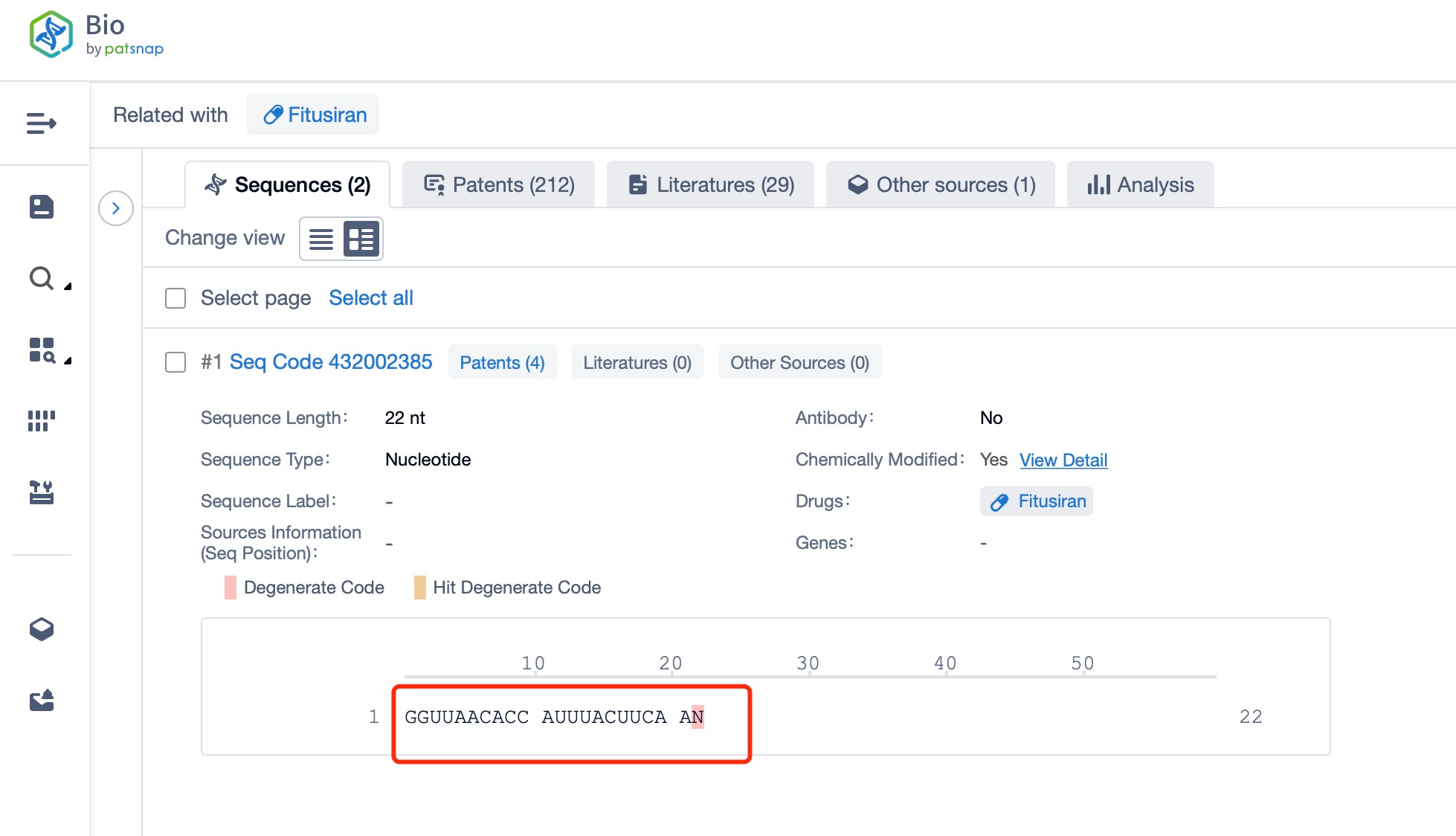
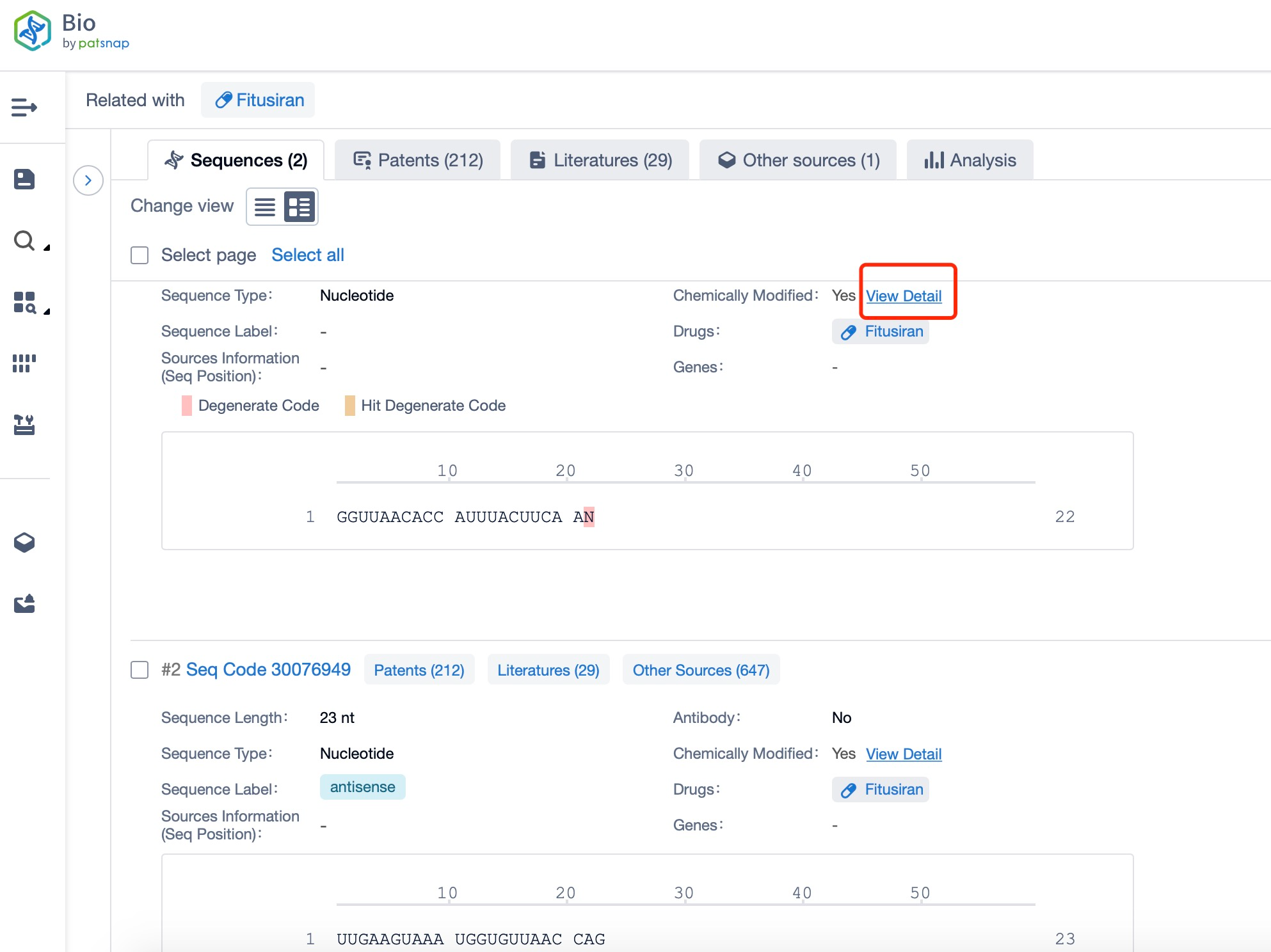
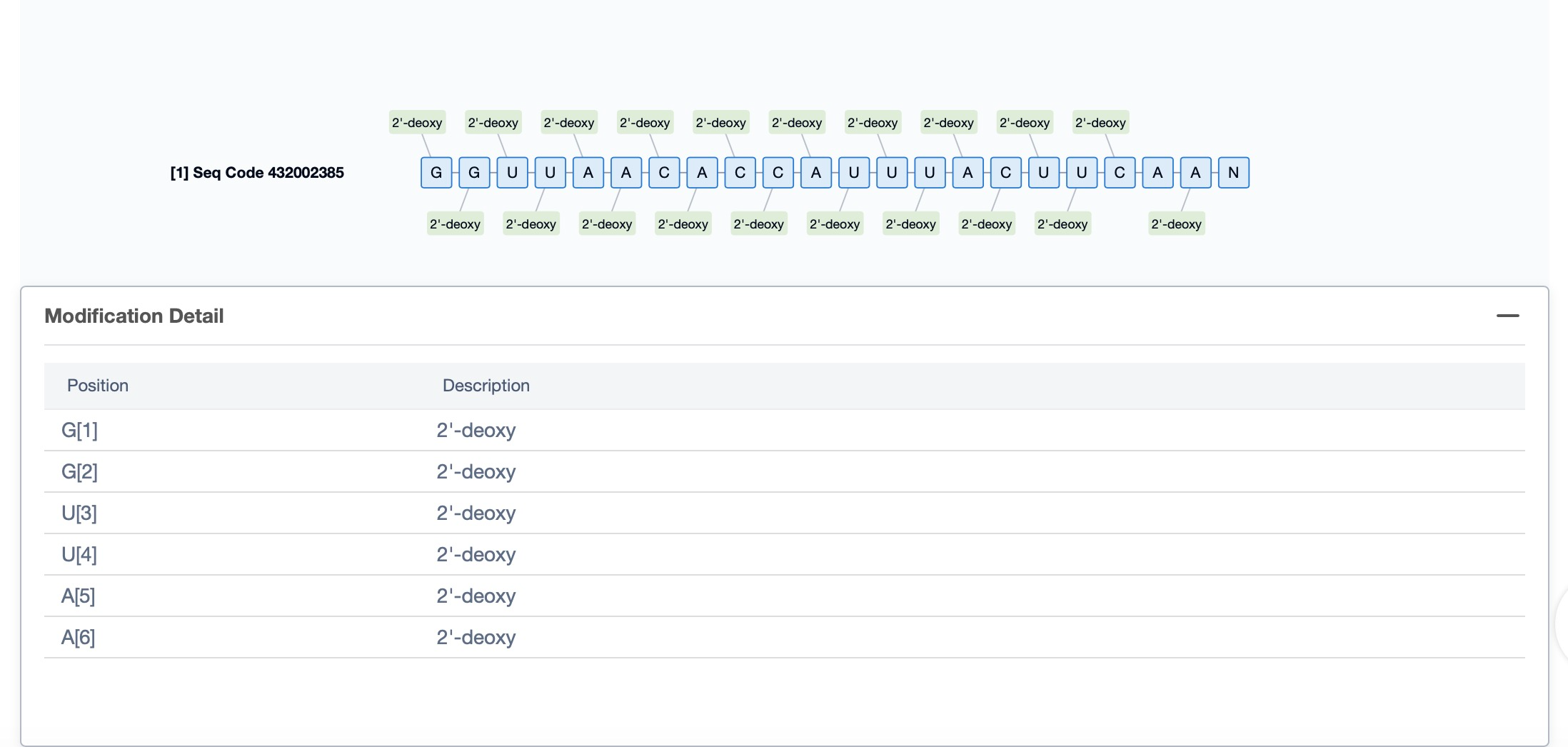
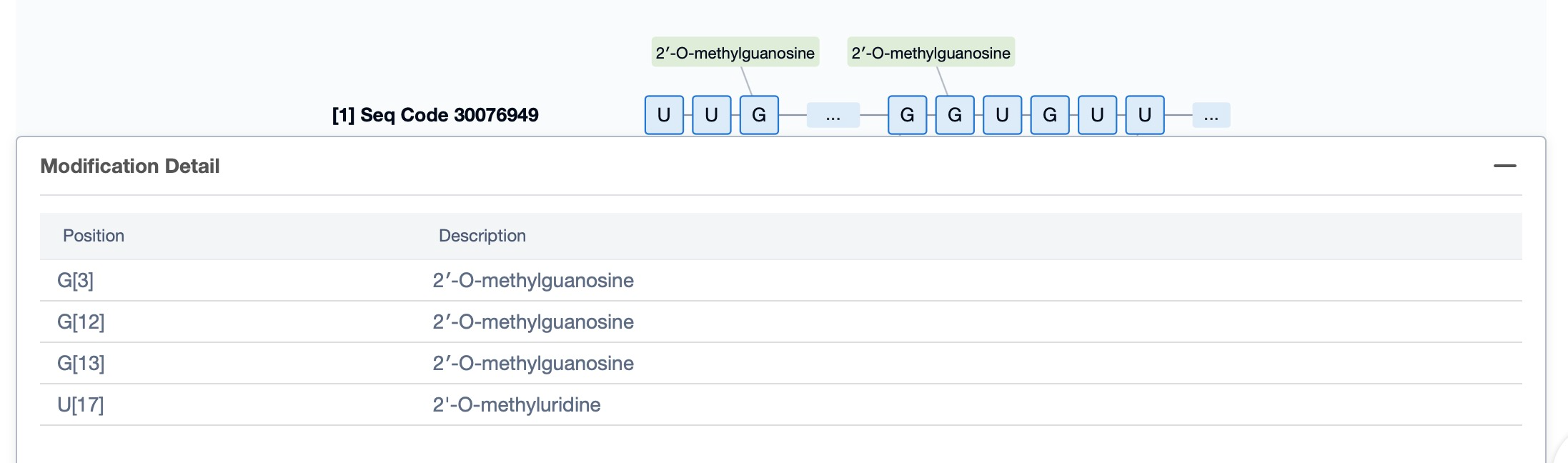
Regarding the sequence information and characteristics of fitusiran, it is a fully modified double-stranded siRNA of 21 and 23 nucleotides long. The drug incorporates a tri-GalNAc-conjugate to facilitate liver-targeted delivery. Specifically, fitusiran contains 21 2'-F substitutions, 23 2'-OMe substitutions, and six phosphorothioate (PS) modifications at the strand ends. These modifications are crucial for enhancing the stability and efficacy of the siRNA in vivo.
Chemical Modification and Action of fitusiran
The chemical structure of fitusiran is designed to optimize its pharmacokinetic and pharmacodynamic properties. The 2'-F and 2'-OMe modifications improve nuclease resistance and reduce immunostimulatory effects, while the PS modifications enhance cellular uptake and tissue distribution. The tri-GalNAc conjugate is particularly important as it enables specific targeting to hepatocytes through the asialoglycoprotein receptor, thereby increasing the efficiency of AT silencing in the liver.
These chemical modifications work synergistically to address several challenges associated with siRNA therapeutics, including stability, off-target effects, and delivery. The 2'-F modifications have been shown to significantly enhance the potency and duration of gene silencing effects. However, the use of extensive chemical modifications also necessitates careful evaluation of potential toxicities, as some modifications can affect the pharmacological properties and safety profile of the siRNA.
Summary and Prospect
The development of fitusiran represents a significant advancement in the field of RNAi therapeutics and hemophilia treatment. Current achievements include the demonstration of sustained AT reduction and improved thrombin generation in clinical trials, which have translated into meaningful reductions in bleeding events for hemophilia patients. The subcutaneous administration route and potential for less frequent dosing compared to factor replacement therapies are also notable advantages.
Looking towards the future, several key areas of research and development are likely to be pursued. First, long-term safety data will be crucial to fully understand the risk-benefit profile of fitusiran, particularly concerning thrombotic events. Optimization of dosing regimens to maintain efficacy while minimizing potential risks is an ongoing area of investigation.
Further research may also focus on identifying biomarkers or patient characteristics that can predict response to fitusiran, enabling more personalized treatment approaches. Additionally, studies comparing fitusiran to other novel hemophilia therapies, such as emicizumab or gene therapies, will be important to define its place in the evolving treatment landscape.
The potential application of fitusiran in other bleeding disorders beyond hemophilia A and B is another area for future exploration. Preclinical studies have suggested potential benefits in rare bleeding disorders such as factor V, VII, and X deficiencies.
As the field of RNAi therapeutics continues to advance, improvements in siRNA design and delivery technologies may further enhance the efficacy and safety profile of drugs like fitusiran. Research into novel chemical modifications or delivery systems could lead to next-generation RNAi therapies with improved target specificity, reduced off-target effects, and enhanced tissue distribution.
In conclusion, fitusiran represents a promising new approach to hemophilia treatment that leverages the power of RNAi technology. Its unique mechanism of action, targeting a common point in the coagulation cascade, offers the potential for a universal hemophilia therapy regardless of factor deficiency or inhibitor status. While challenges remain, particularly in terms of long-term safety, the development of fitusiran has already contributed significantly to our understanding of siRNA therapeutics and their application in treating genetic disorders. As clinical trials progress and more data becomes available, fitusiran may emerge as an important addition to the armamentarium of treatments for hemophilia and potentially other bleeding disorders. The ongoing research and development in this field underscore the transformative potential of RNAi-based therapies in addressing previously intractable medical challenges.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
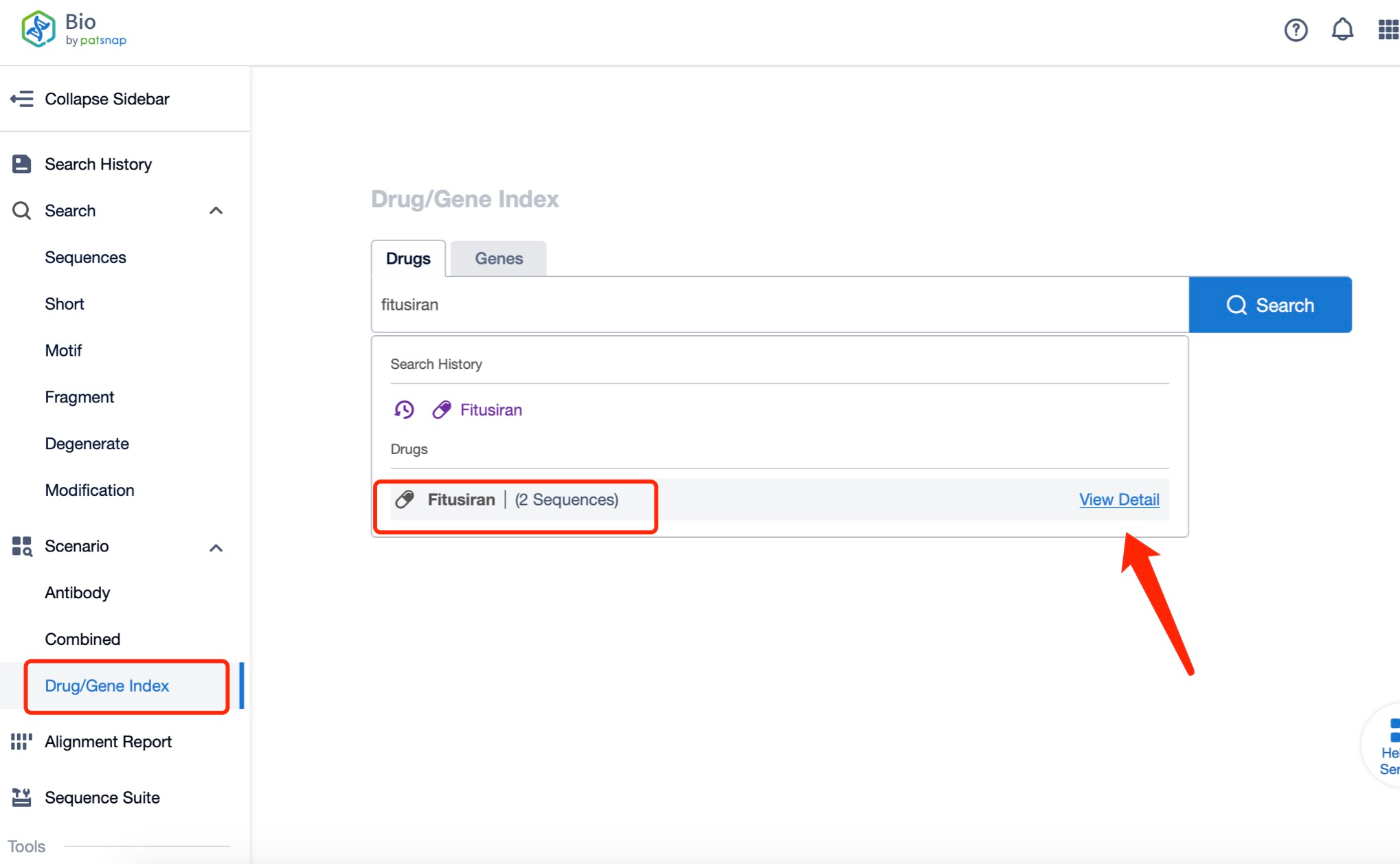
Taking fitusiran as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' fitusiran ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of fitusiran.
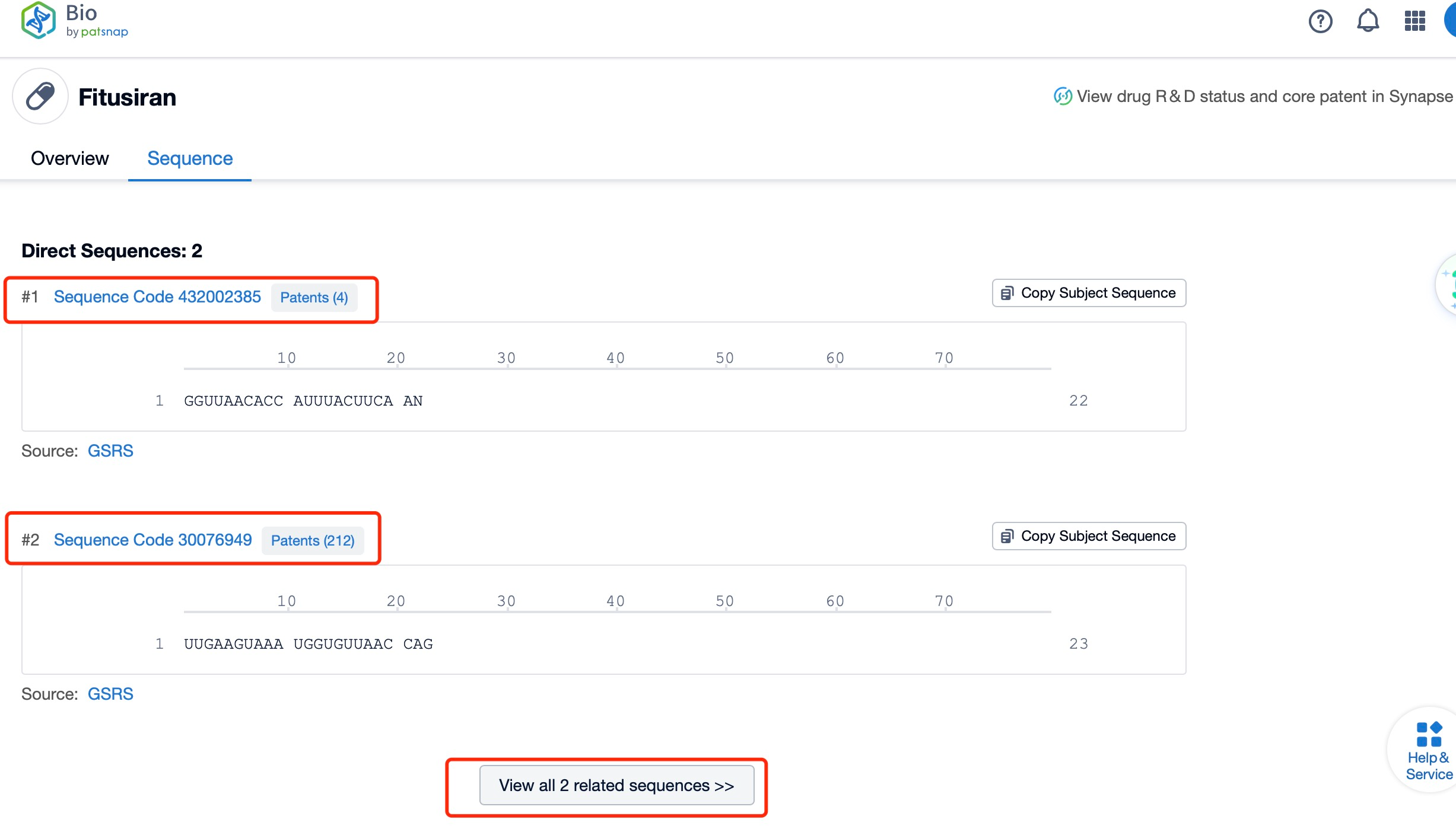
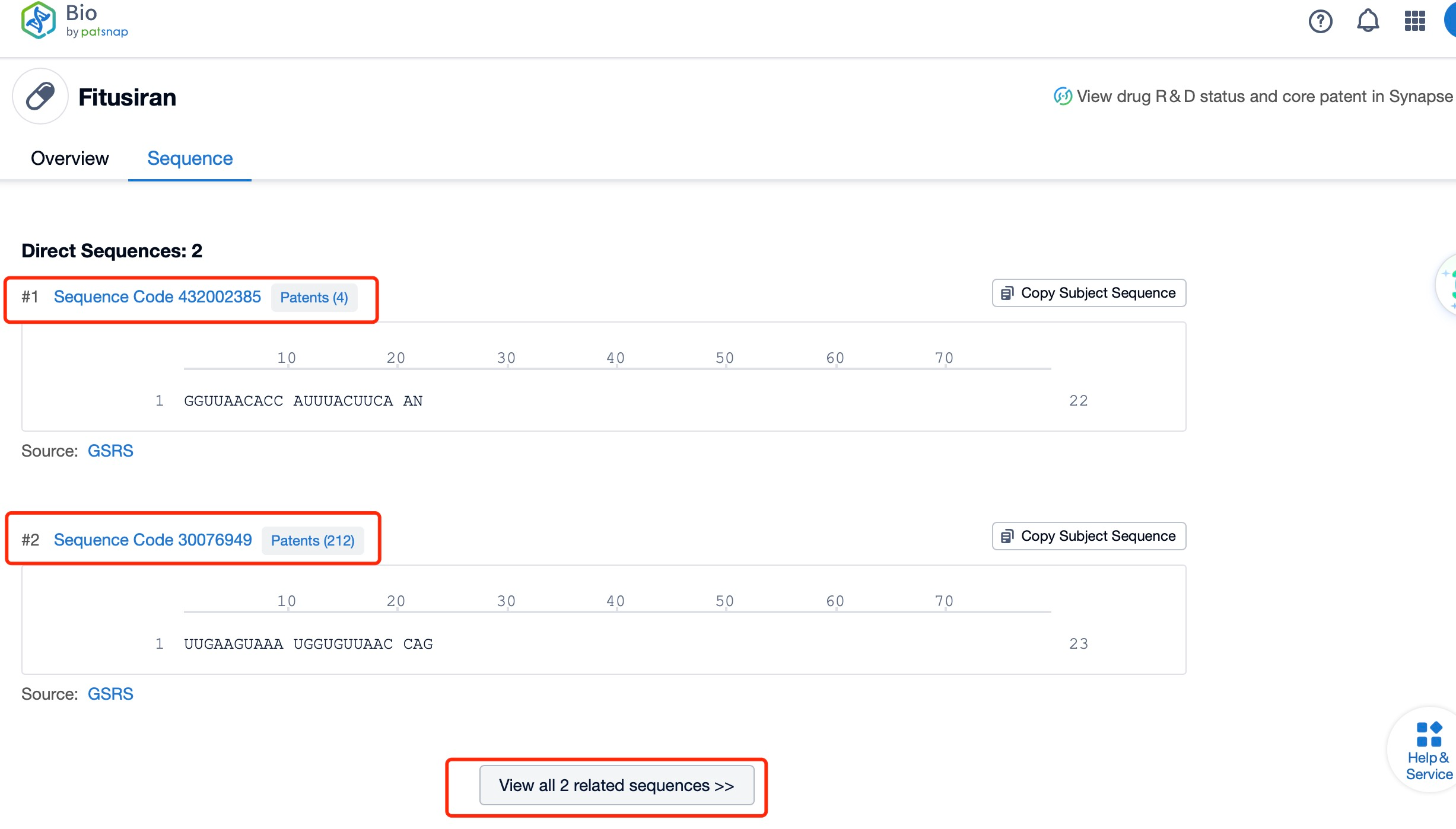
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.