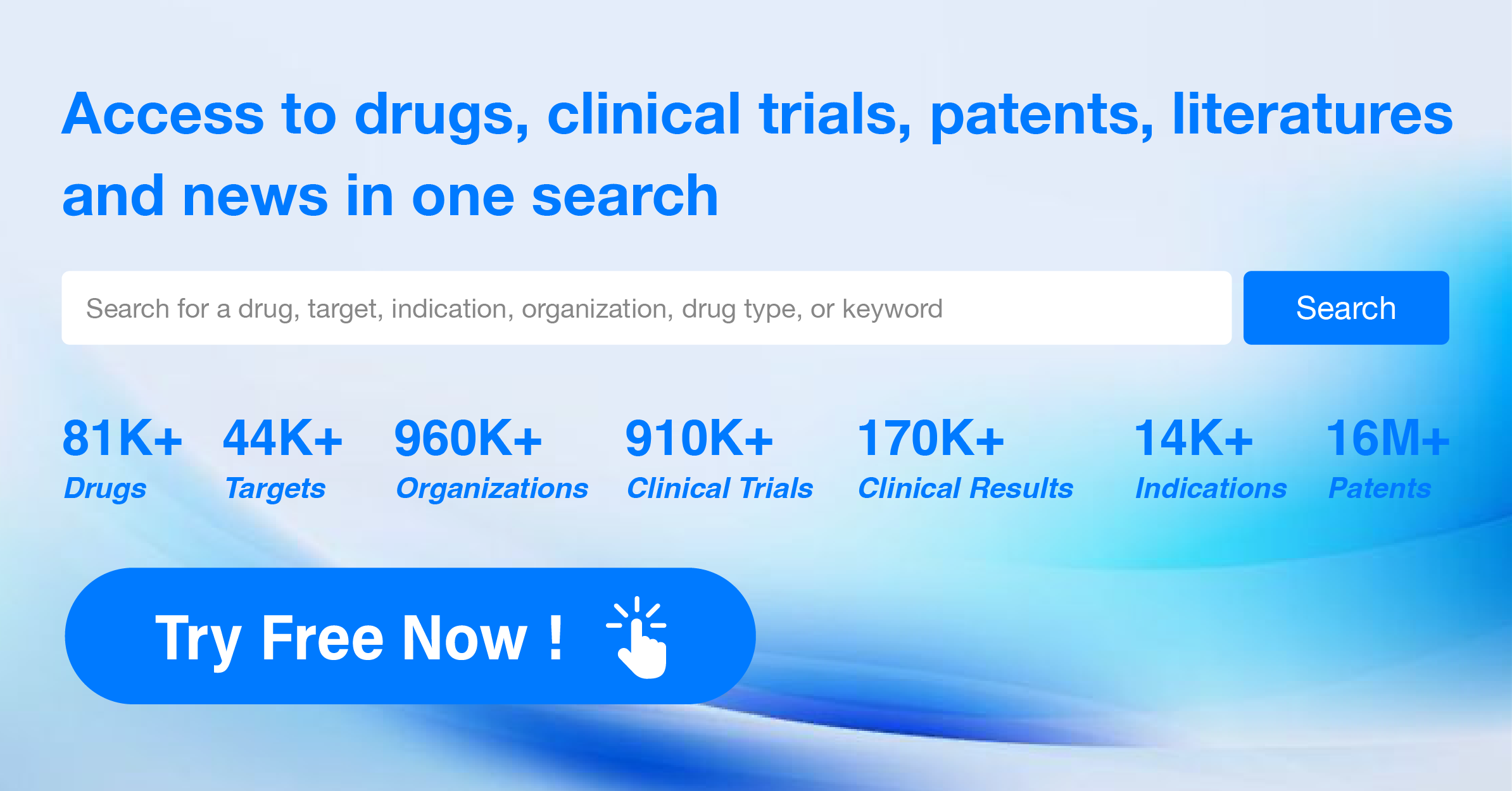
How to Obtain Global New Drug Research and Development Trends?
Several approaches can be employed to swiftly follow the latest global developments in new drug research and development:
1.Subscribe to industry journals and news: Journals frequently publish the latest information on new drug research and regulatory approvals.
2.Track updates from regulatory agencies: Regulatory agencies such as the FDA, EMA, and NMPA regularly update their official websites with news announcements, approval statuses, and clinical trial recruitment.
3.Specialized databases and information services: Websites that provide professional pharmaceutical information data summarization and analysis, such as ClinicalTrials.gov and the Wisdom Pharmaceutical New Drug Intelligence Database.
4.Attend industry conferences and webinars: Participating in these events provides access to the most cutting-edge research information and opportunities for interaction with industry experts.
5.Social media and discussion groups: Follow relevant industry leaders and organizations on LinkedIn and Twitter. Using social media can quickly yield insights into industry trends from professionals.
6.Industry reports and analyst reports
7.Utilize news aggregation tools: Tools like Feedly and Google Alerts can help you customize subscriptions to news and articles on topics of interest.
8.Join professional online forums and communities for exchanges and discussions with industry peers.
9.Academic institutions and research organizations: Pay attention to information released by top universities and research institutions, which can provide updates on research progress and new discoveries.
By employing the methods listed above, you can ensure you are quickly updated and aware of global developments in new drug R&D.
It is especially recommended to use the Synapse database, which offers a comprehensive and daily updated repository of global medical data and is free to register and use.