Macomics Unveils Breakthrough Anti-LILRB Antibody at 2024 AACR with Promising Preclinical Data
Macomics Ltd, at the forefront of exploratory macrophage-targeted therapeutics, has revealed specifics about its premier project, a novel class of monoclonal antibody that functions regardless of ligand and targets pan-LILRB, designed as a cancer therapy. During the American Association for Cancer Research's yearly conference, held in San Diego, the company showcased pivotal findings related to their drug candidate, MACO-355.
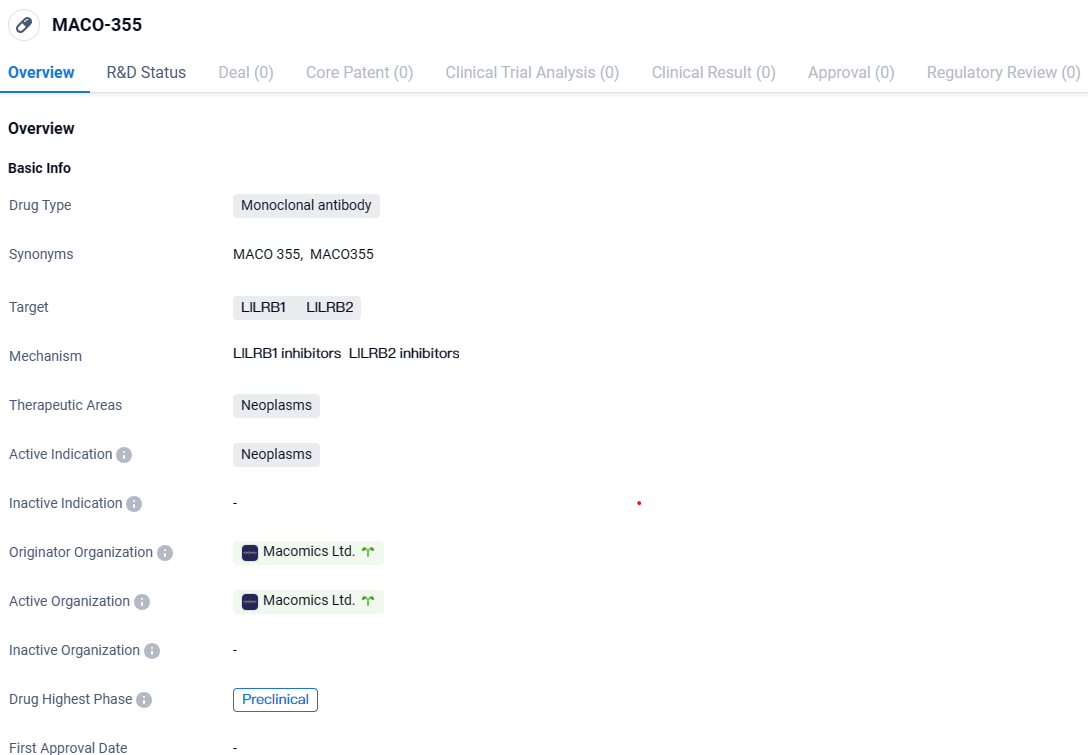
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MACO-355 represents an innovative therapeutic development, characterized as a broad-spectrum, non-blocking antibody specifically designed to engage with multiple LILR proteins. Its primary function is to alter macrophage behavior in a manner that overcomes immunosuppressive environments. Distinctively, it does not impede the connection between LIL-receptors and the MHC Class I molecules, making it the first antibody of its kind to demonstrate such independence from ligand interaction. This unique manner of operation enhances the functionality of the antibody, as evidenced by its efficacy in altering macrophage behavior and stimulating T cell response.
In addition to these capabilities, MACO-355 promotes the engulfment of cancer cells by macrophages and the destruction of these cells by NK cells in a manner unaffected by the presence of ligands, setting it apart from antibodies that rely on ligand obstruction. Comparative studies place MACO-355 in a league of its own, highlighting its singular capacity to activate macrophages even in highly immunosuppressive contexts. Experimental findings further revealed MACO-355's potential to counteract the immunosuppressive effects perpetrated by M2 macrophages and to impede the progression of tumors both in vitro and in live models.
MACO-355 selectively latches onto LILRB members and targets a distinctive epitope close to the cell surface. It demonstrates full efficacy only upon the interaction with Fc receptors, and it intricately alters the macrophage's internal kinase network through its distinctive mechanism of action. The team at Macomics theorizes that the ligand-independent functionality coupled with its proficiency in activating severely suppressed macrophages could translate into superior clinical efficacy and pave the way for patient selection based on biomarkers.
Macomics' CEO, Stephen Myatt, declared the company's strategic focus on the progression of MACO-355, with aspirations to swiftly propel this groundbreaking antibody through the therapeutic pipeline as a means to enhance cancer patient survival over the long term. Concurrently, Macomics is employing its proprietary discovery platform to explore therapeutic solutions for fibrosis and inflammation, convinced that targeting macrophage subsets specific to these conditions could offer revolutionary treatment modalities to better patient prognoses.
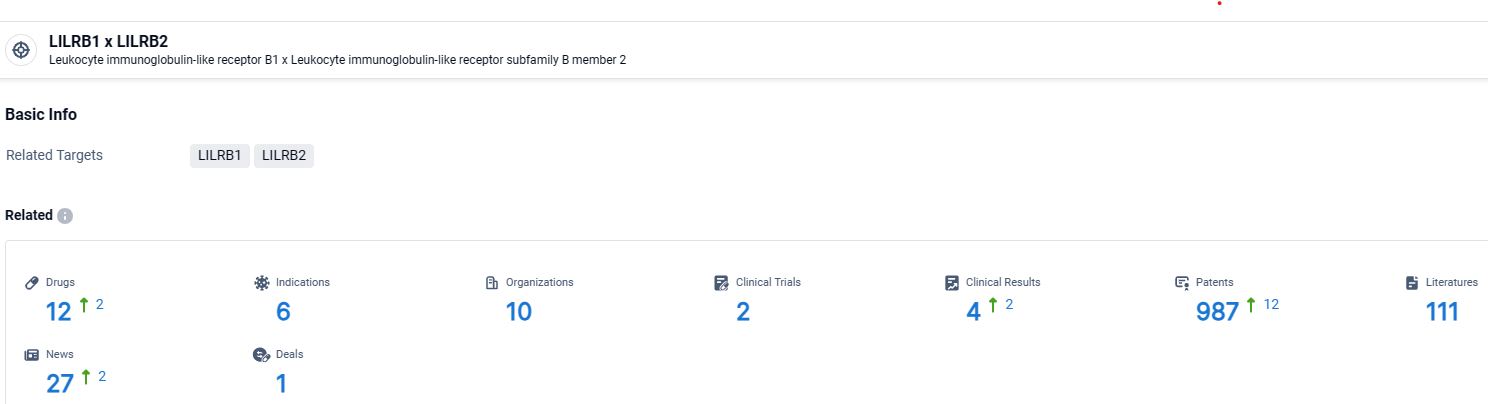
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 15, 2024, there are 12 investigational drugs for the LILRB1 and LILRB2 and albumin target, including 6 indications, 10 R&D institutions involved, with related clinical trials reaching 2, and as many as 987 patents.
MACO-355 targets the proteins LILRB1 and LILRB2 and is indicated for the treatment of neoplasms. Currently in the preclinical phase, MACO-355 is still undergoing laboratory testing and has not yet progressed to human clinical trials.