Tallac Therapeutics Presents TAC-001 and Cancer Vaccine Findings at AACR 2024
Tallac Therapeutics, Inc., an emerging force in the biopharmaceutical industry with a focus on proprietary antibody-oligonucleotide conjugate technology, recently unveiled preclinical findings showcasing the ability of their investigational compound, TAC-001, to significantly enhance the effectiveness of therapeutic cancer vaccines and revive previously diminished immune responses to such vaccines.
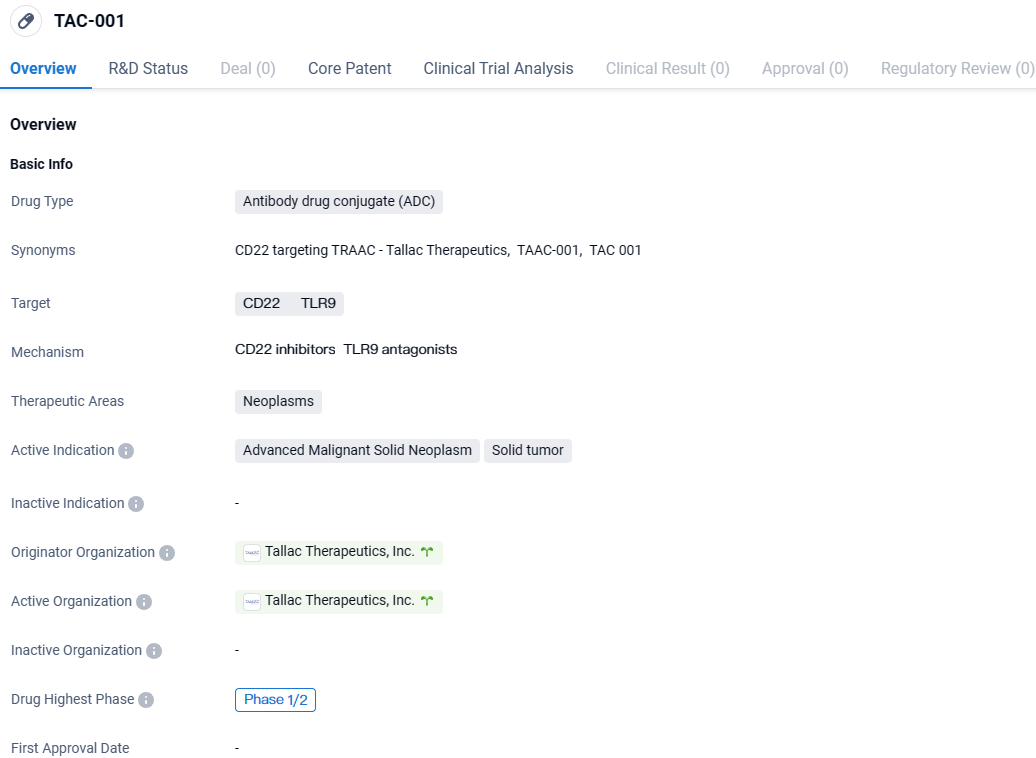
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Currently under investigation, TAC-001 is a cutting-edge therapeutic, designed as a Toll-like Receptor Agonist Antibody Conjugate that is administered systematically. It specifically targets B cells to provoke an immune response against cancer. This research will be shared in the session named "Vaccines, Antigens, and Antigen Presentation 2" during the American Association for Cancer Research Annual Meeting of 2024, from April 5 to April 10 in San Diego.
The study titled "A B cell targeted TLR9 Agonist Antibody Conjugate Enhances the Potency of Cancer Vaccines and Revitalizes Immune Function in Seniors" will be displayed in poster form. It reveals that TAC-001, when used alongside cancer vaccines, not only induces strong and lasting IgG antibody levels aimed at the vaccine but also influences the humoral immune reaction towards a Th1 bias and restores immune responses that typically wane with age. In addition, TAC-001 intensifies T cell responses and their ability to kill cancer cells, thus significantly upgrading the performance of anti-cancer vaccines.
Dr. Hong I. Wan, who serves as the president, CEO, and one of the founders of Tallac Therapeutics, stated: “The insightful data on TAC-001's synergistic effects with cancer vaccines deepen our grasp on how TAC-001 mobilizes both the humoral and cellular branches of anti-cancer immunity. These findings strongly support its clinical use alongside cancer vaccines. The preclinical validation of this concept is promising, and it opens new avenues for TAC-001’s application not just in cancer treatment but also for other pathological conditions.”
The molecular structure of TAC-001 involves a powerful TLR9 agonist linked to an antibody that targets CD22, which is a B cell-specific receptor that also appears on cancer-infiltrating B cells. The purpose of TAC-001 is to directly transport T-CpG to B cells via CD22 attachment, which prompts TAC-001 to be taken into the cells, activating TLR9 signaling and B cell activation. This chain reaction ignites a series of immunological responses. Preclinical evaluations show that the resulting innate and adaptive immune responses triggered by TAC-001 exhibit profound anti-cancer capabilities.
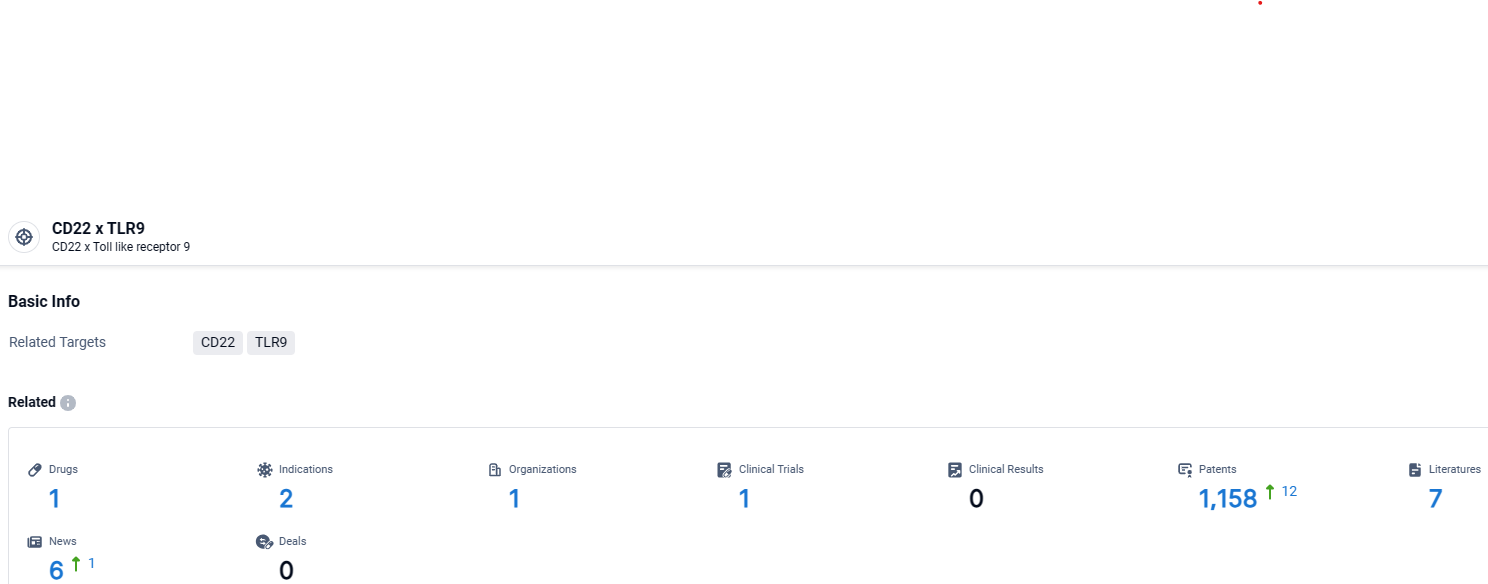
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 15, 2024, there are 1 investigational drugs for the CD22 and TLR9 target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 1158 patents.
TAC-001 targets CD22 and TLR9 and is focused on the therapeutic areas of neoplasms, specifically advanced malignant solid neoplasms and solid tumors. The drug is currently in Phase 1/2 of clinical development, indicating that it has undergone initial testing in humans. Further research and clinical trials will be needed to determine the safety and efficacy of TAC-001.